Business Consulting for Healthcare and Life Sciences
Clarity in complexity. Confidence in decisions. Control through evidence.
At GxPVigilance, our business consulting for healthcare and life sciences organisations helps strengthen governance, improve efficiency, and align strategic decisions with regulatory and financial integrity.
We provide an independent, data-driven perspective that turns compliance and financial challenges into actionable strategies — helping leaders build clarity and control across operations.
At GxPVigilance, our business consulting for healthcare and life sciences organisations helps strengthen governance, improve efficiency, and align strategic decisions with regulatory and financial integrity.
We provide an independent, data-driven perspective that turns compliance and financial challenges into actionable strategies — helping leaders build clarity and control across operations.
Why Business Consulting Matters
In regulated healthcare environments, success depends on more than compliance. It requires systems that are financially sustainable, operationally sound, and strategically aligned.
Our consulting services help leaders identify inefficiencies, assess risk, and make informed decisions supported by evidence, not assumptions.
Our consulting services help leaders identify inefficiencies, assess risk, and make informed decisions supported by evidence, not assumptions.
We partner with executives, quality teams, and clinical leaders to:
- Assess the health of operations across finance, quality, and governance.
- Identify structural gaps that affect performance or compliance.
- Build realistic roadmaps for sustainable improvement.
- Strengthen internal decision frameworks before inspections or restructuring.
Our approach combines the precision of ISO-aligned auditing with the strategic foresight of management consulting. We enable progress through partnership, not perfection.
Our Consulting Scope
Operational Gap Assessment
Our life sciences business consulting services go beyond traditional audits — we integrate operational, financial, and governance insights to help regulated organisations build clarity and measurable progress.
We benchmark your practices against ICH, TGA, NHMRC, ISO 9001, and other standards, delivering prioritised recommendations.
We benchmark your practices against ICH, TGA, NHMRC, ISO 9001, and other standards, delivering prioritised recommendations.
Key focus areas:
- We map processes and optimise resources
- SOP structure, ownership, and accountability
- Data delays and rework raise costs and extend timelines. We implement workflow automation and data integrity, streamlining operations and boosting efficiency.
- Leadership and role clarity within regulated teams
- Governance alignment and readiness for scale
Each engagement concludes with a Gap-to-Goal Matrix, a visual roadmap that clarifies actions, ownership, and solutions.
Financial and Performance Review
Through our business consulting for healthcare clients, we uncover the root causes of financial inefficiency and help restore balance between compliance obligations and financial sustainability.
Typical outcomes include:
- Transparent trial- or project-level P&L structures
- Revenue recognition and cash-flow improvement plans
- Cost attribution models linking effort to value
- Working capital optimisation and forecasting
- Strategic margin thresholds and sponsor negotiation frameworks
These reviews provide executives with the visibility and control necessary for informed decisions.
Strategic and Organisational Review
When leaders require direction or structure, we conduct independent strategic reviews that combine operational, governance, and financial insights.
We help boards and executives look past symptoms, pinpointing root causes and priority actions.
We help boards and executives look past symptoms, pinpointing root causes and priority actions.
We analyse:
- Organisational design and leadership coverage
- Vendor and partner dependencies
- Governance and reporting frameworks
- Change readiness and communication pathways
We deliver an executive summary, a 12-month roadmap, and a prioritised transformation plan that balances urgency and capacity.
Change Management and Capability Building
Every improvement plan requires people to deliver.
We embed change management and training into every engagement, making recommendations practical and achievable.
We embed change management and training into every engagement, making recommendations practical and achievable.
Our support includes:
- Transition plans that minimise disruption
- Staff capability assessments and targeted upskilling
- Governance workshops to align leadership on priorities
- Templates for performance tracking and reporting
Progress means fixing issues and bringing your team along.
Our Consulting Approach: Evidence Before Opinion
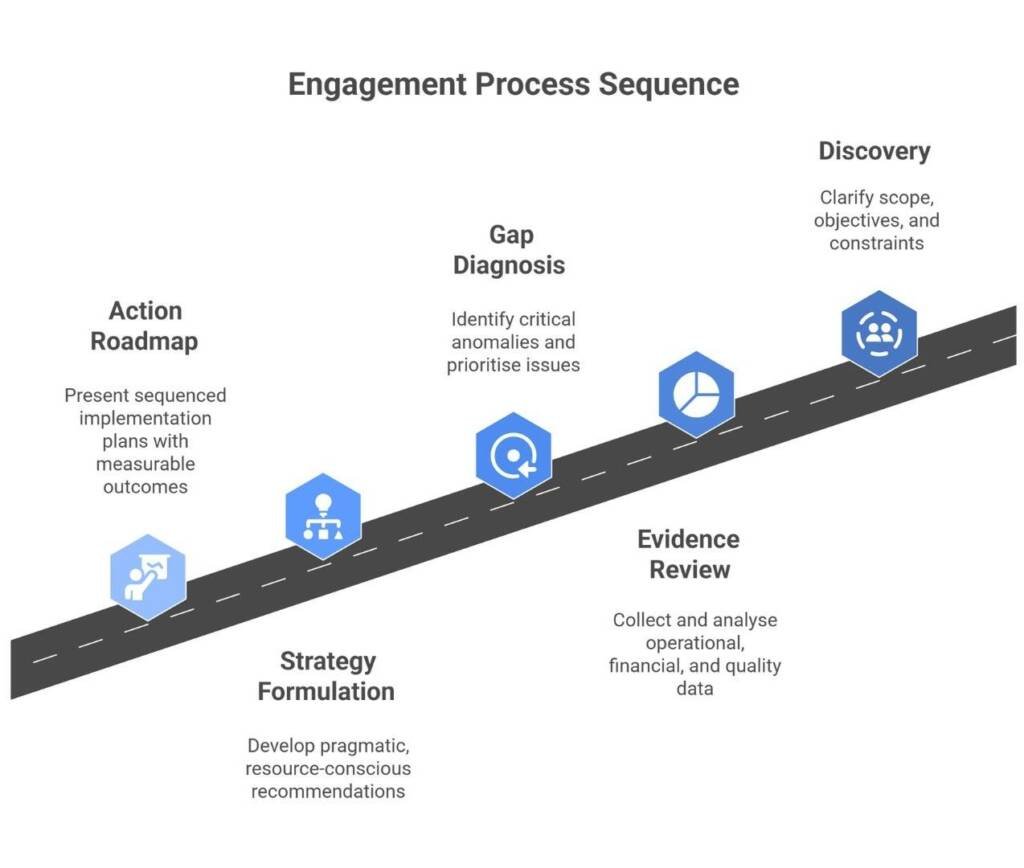
All work is conducted under our ISO 9001-aligned Quality Management System, ensuring consistency, traceability, and defensible evidence for every conclusion.
Who We Serve
We work with organisations where regulatory precision meets operational complexity, including:
- Clinical trials units and research institutes
- Pharmaceutical and biotechnology sponsors
- Hospitals and healthcare networks
- Pharmacovigilance and regulatory service providers
- Not-for-profit and public sector research entities
Whether you are restructuring, scaling, or seeking assurance, we help turn insight into clarity and oversight into control.
Why Choose GxPVigilance
- Independent and Evidence-Based: Objective insight grounded in financial, operational, and regulatory data.
- Integrated Perspective: We bridge clinical, quality, and commercial systems, connecting compliance with business performance.
- Actionable Results: Recommendations are sequenced and achievable within your current resource framework.
- Qualified Expertise: Our consultants are certified auditors (ISO 9001, ISO 42001, ISO 13485) with extensive experience in healthcare and regulatory compliance.
Explore our related services:
• GVP Auditing Services – ensuring ongoing patient safety through system assurance.
• GCP Clinical Trial Auditing – protecting data integrity and participant wellbeing.
• Ethics & Governance Auditing – upholding research credibility and compliance.



