Introduction
Australian QPPVs and A-PVCPs now face a challenging environment.The modern Australian QPPV in 2026 works under the same TGA responsibilities, but the methods, tools, and expectations are changing fast. QPPVs remain accountable for oversight and, in many organisations, are still responsible for case processing, signal detection, PSURs, and inspection readiness—even as AI systems reshape how this work is done.
TGA responsibilities are constant, but methods are changing fast. QPPVs remain accountable for oversight; in some situations, they are responsible for case processing, signal detection, PSURs, and inspection readiness. AI tools now screen thousands of abstracts in minutes, generate case narratives, and flag safety signals sooner than traditional methods.
Regulators ask: How are these systems validated? Who is accountable for AI errors? How does human oversight fit in?
I’m Carl Bufe—practising pharmacist, auditor, working QPPV, and someone who’s spent the last three years rebuilding regulatory, operational and pharmacovigilance workflows with AI while keeping them audit-ready. Here’s what actually matters for Australian QPPVs in 2026 —and what you can safely ignore while the hype settles.
What the TGA Still Requires: Your Foundation Hasn’t Changed
Australian QPPVs / QPPVAs (often serving dual roles as the Australian Pharmacovigilance Contact Person or A-PVCP) must reside in Australia and maintain robust oversight of the sponsor’s entire pharmacovigilance system. This may include ensuring timely reporting of Individual Case Safety Reports (ICSRs), managing Significant Safety Issues (SSIs), overseeing Periodic Safety Update Reports (PSURs), and maintaining inspection readiness.
While medical qualifications aren’t mandatory, the TGA strongly recommends access to medically qualified personnel for clinical assessments—ideally residing in Australia and registered locally to provide context-relevant clinical judgment. The QPPV must possess extensive training in Australian pharmacovigilance regulations and demonstrate a deep knowledge of TGA requirements, which differ substantively from those of the EU and the US.
Core responsibilities remain:
- Ensuring effective oversight
- Ensuring appropriate medical review of cases
- Maintaining compliant adverse event collection, assessment, and reporting systems
- Overseeing signal management and risk minimisation activities
- Keeping your pharmacovigilance system inspection-ready
- Demonstrating comprehensive expertise in fundamental pharmacovigilance principles
AI doesn’t change these obligations, at the moment, only how efficiently you can meet them—if properly controlled.
The AI Shift: What’s Actually New (And What Isn’t)
The transformation isn’t that AI exists; it’s that AI exists. It’s that AI is now accurate, fast, and cheap enough to handle tasks that previously required extensive manual effort.
What AI systems now achieve:
- Literature screening with 90-95% detection rates that now exceed traditional manual methods
- Case narrative generation from structured fields
- Pattern identification across millions of safety records through machine learning models
- Adverse event extraction from unstructured medical reports using natural language processing (NLP)
- Automated case processing and signal detection
What AI cannot replicate:
- Final causality determinations—these remain human responsibilities
- Clinical judgment for verifying links between drugs and adverse events
- Interpreting narrative reports with contextual nuances, idioms, and subtleties that even advanced NLP struggles to capture perfectly.
- Regulatory accountability (you’re still signing the documents)
As documented in industry analysis, “AI won’t replace professionals. But professionals who use AI will replace those who don’t.”
The challenge is not whether to use AI, but how to implement it safely and in compliance with TGA expectations.
The Technical Skills That Actually Matter
You do not need to become a data scientist, but you must possess clear, practical AI literacy. This means understanding how AI models are used in pharmacovigilance work, interpreting their outputs, and identifying and managing potential errors or biases in AI-assisted workflows.
1. Conceptual Understanding of AI Systems
You need a conceptual understanding of machine learning, natural language processing (NLP), and deep learning architectures that now automate case processing, narrative generation, signal detection, and literature surveillance.
Understanding how these tools work enables you to:
- Validate outputs through a clinical and regulatory lens.
- Identify algorithmic errors when they occur.
- Understand model limitations
- Initiate retraining when performance degrades.
- Implement appropriate guardrails for AI systems, particularly large language models (LLMs) used in high-stakes safety tasks.
QPPVAs must ensure that AI outputs remain traceable, explainable, and safe, in line with Good Machine Learning Practice (GMLP) principles. This requires understanding explainable AI (XAI) techniques like SHAP and LIME that make black-box model predictions interpretable to regulators and medical reviewers.
2. Validation Frameworks for AI Systems
Modern QPPVAs must master AI validation frameworks that extend beyond traditional software validation. The pharmaceutical industry increasingly applies GAMP 5 Second Edition principles to AI systems, requiring risk-based validation throughout the AI lifecycle.
Regulatory frameworks from the FDA, EMA, TGA, and CIOMS Working Group XIV now mandate specific validation approaches for AI in pharmacovigilance. The FDA’s 2025 draft guidance on AI credibility assessment frameworks, the EMA’s AI workplan, which emphasises transparency and continuous monitoring, and CIOMS guidance on responsible automation all require QPPVAs to demonstrate competency in AI oversight mechanisms.
Your validation framework must address:
- How to document AI system architecture
- Establishing performance monitoring protocols
- Maintaining validation status as models evolve
- System registers showing AI classification logic.
- Audit trails tracing how AI models classify cases, what algorithm versions were deployed, and with what confidence levels
The TGA’s Pharmacovigilance Inspection Program (PVIP) increasingly assesses these AI governance elements during audits.
3. Data Science and Analytical Competencies
You should be able to read and interpret key AI-generated outputs, such as model confidence scores, statistical findings, and flagged safety signals. A basic understanding of predictive analytics and common statistical principles is essential for contextualising these outputs and making sound regulatory decisions.:
- Predictive analytics for risk assessment
- Disproportionality analysis augmented by machine learning signal-detection methodologies that simultaneously mine electronic health records, claims data, social media, and literature.
Real-world evidence (RWE) capabilities are increasingly critical. Modern pharmacovigilance extends beyond spontaneous reporting to encompass proactive monitoring of on-market medicine performance using diverse real-world data sources. QPPVAs must understand how to integrate and analyse data from patient registries, EHRs, wearable devices, and social media platforms to detect safety signals before they emerge through traditional channels.
4. Data Governance and Bias Detection
Data governance and privacy competencies are paramount. QPPVAs must navigate GDPR compliance, understand federated learning approaches that enable collaborative AI training without data sharing, and ensure data quality, integrity, and bias mitigation across diverse datasets.
Understanding how AI models might perpetuate demographic or reporting biases—potentially missing real risks in underrepresented populations—is essential for patient safety.
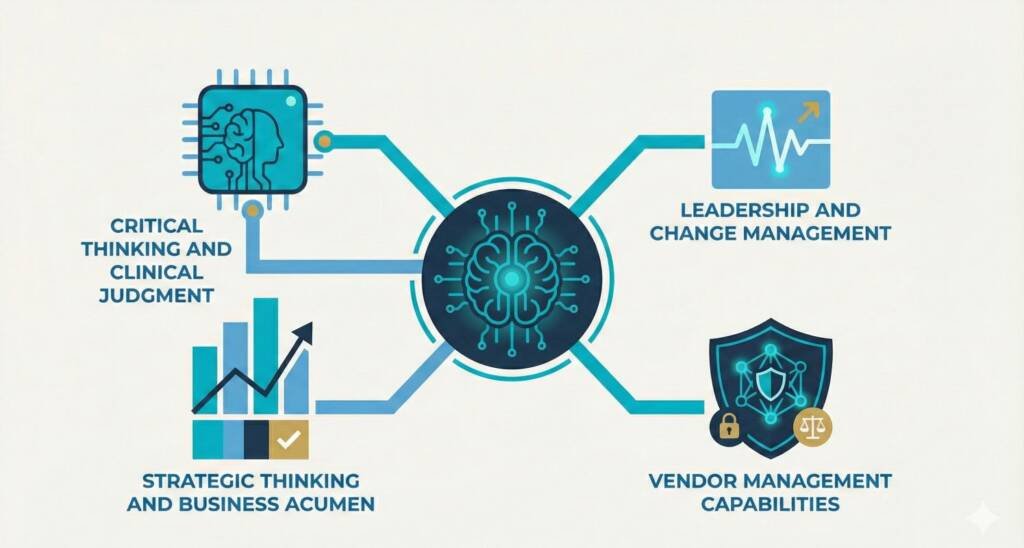
The Human Skills That Matter More Than Ever
As automation handles routine tasks, QPPVAs must excel at higher-order cognitive functions that AI cannot replicate.
1. Critical Thinking and Clinical Judgment
Final causality decisions remain human responsibilities—AI can flag potential signals, but seasoned clinical judgment must verify links between drugs and adverse events. This requires interpreting narrative reports with their contextual nuances, idioms, and subtleties that even advanced NLP struggles to capture perfectly.
Ethical oversight and bias detection capabilities distinguish effective QPPVAs in the AI era. They must ensure patient safety isn’t driven solely by automation but by informed human judgment that recognises when AI outputs require sceptical review. This includes monitoring for algorithmic bias, demanding explainable AI, and maintaining awareness of model limitations and failure modes.
2. Leadership and Change Management
Modern QPPVAs function as transformation leaders, not merely compliance officers. They must guide AI adoption across multidisciplinary teams, managing workforce transitions as case processors evolve from manual data entry to validating AI outputs and training systems through feedback loops.
This requires applying change management frameworks, such as Rogers’ Diffusion of Innovation Theory, to categorise team members (Innovators, Early Adopters, Early Majority, Late Majority, Laggards) and tailor engagement strategies accordingly.
3. Strategic Thinking and Business Acumen
Strategic thinking and business acumen separate exceptional QPPVAs from adequate ones. They must translate regulatory requirements into business value propositions, articulate how AI investments deliver 50-80% time savings and 30-50% cost reductions, and align safety strategies with organisational objectives.
This includes building business cases for AI implementation, demonstrating ROI, and communicating technical concepts to executive leadership in financial and strategic terms.
4. Vendor Management Capabilities
As pharmaceutical companies outsource 60%+ of pharmacovigilance functions to specialised providers, vendor management capabilities are increasingly critical. QPPVAs must evaluate AI-enabled service providers, conduct risk-based vendor assessments, establish governance frameworks, and maintain oversight through performance metrics and CAPA plans.
Interdisciplinary Collaboration and Communication Excellence
The complexity of AI-augmented pharmacovigilance demands exceptional collaboration skills. QPPVAs must bridge worlds between clinicians, data scientists, AI developers, regulatory professionals, and business stakeholders.
This requires translating technical AI concepts into regulatory language for TGA inspectors, explaining safety signals to non-technical executives, and coordinating feedback loops between pharmacovigilance teams and algorithm developers.
Stakeholder communication extends to patients, healthcare professionals, and advocacy groups. As pharmacovigilance moves toward patient-centric approaches emphasised in CIOMS guidance, QPPVAs must engage diverse voices in safety monitoring and ensure AI systems don’t perpetuate health inequities.
Emotional intelligence and soft skills emerge as differentiators. With AI handling analytical tasks, human skills like empathy, self-awareness, motivation, and social adeptness become paramount. Communication skills and emotional intelligence consistently rank as the most critical attributes (mean ratings 4.8 and 4.7 out of 5), followed by negotiation, problem-solving, and adaptability.
Regulatory Intelligence and Continuous Learning
Modern QPPVAs must master regulatory intelligence capabilities that extend beyond reactive compliance. This involves systematically monitoring global regulatory developments from the TGA, FDA, EMA, MHRA, and ICH, analysing their implications, and translating trends into actionable strategies.
With AI regulations evolving rapidly—the TGA’s 2025 AI review, FDA’s AI guidance, EMA’s AI workplan, and CIOMS Working Group XIV recommendations—staying current requires dedicated environmental scanning.
Continuous professional development is non-negotiable. QPPVAs must pursue AI-focused certifications, attend specialised training on machine learning in GxP environments, and participate in professional networks that share implementation best practices.
The Australian Context: What’s Specific to Our Market
Australian QPPVAs operate within specific requirements that add context to the broader competency framework:
- Mandatory Australian residency for the QPPV/A-PVCP role
- Understanding TGA-specific requirements that differ substantively from EU/US frameworks
- Building networks within the Australian pharmaceutical ecosystem
- Proactive engagement with the TGA’s evolving AI guidance, particularly regarding software as medical devices (SaMD) and post-market surveillance technology
Emerging Competencies for 2030 and Beyond
Looking toward 2030, additional capabilities will distinguish leading QPPVAs. These include understanding AI agents that autonomously initiate signal-detection workflows, predictive risk modelling using genomic and personalised medicine approaches, blockchain for data integrity and traceability, and adaptive AI systems that continuously learn from new safety data.
QPPVAs will increasingly serve as “strategic stewards of AI tools,” guiding implementation, ensuring accuracy and compliance, and leading rather than merely adapting to technological change. Their roles will encompass hybrid responsibilities—overseeing both traditional pharmacovigilance and AI innovation, maintaining inspection readiness, and driving digital transformation initiatives.
Practical Implementation: Building AI-Ready QPPV Capabilities
Organisations should implement structured approaches to develop these competencies:
Foundation building:
- Launch AI literacy programs covering NLP, machine learning, LLM guardrails, and explainability.
- Embed human oversight into AI validation processes.
- Create cross-functional teams blending pharmacovigilance professionals, data scientists, ethicists, and regulators.
Ongoing development:
- Invest in ongoing education through conferences and certifications.
- Redesign workflows to leverage AI strengths while defining meaningful human roles
- Establish centralised AI inventories that classify each system by risk level and link to responsible oversight teams.
Final Remarks
The modern Australian QPPV role converges traditional pharmacovigilance expertise, advanced AI skills, strategic leadership, and a commitment to continuous learning. While regulatory foundations remain constant—ensuring patient safety through rigorous adverse event monitoring—methods, tools, and skill sets expand exponentially in the AI era.
Success in this transformed landscape demands professionals who combine clinical judgment with data literacy, regulatory knowledge with technical fluency, and compliance rigour with innovation leadership. Those who develop these hybrid capabilities will position themselves and their organisations to leverage AI’s transformative potential while maintaining the human oversight, ethical vigilance, and strategic thinking that remain irreplaceable in safeguarding patient safety.
The question facing Australian pharmaceutical organizations is no longer whether to integrate AI into pharmacovigilance, but how to develop QPPVs with the sophisticated skill sets required to lead this transformation responsibly, compliantly, and effectively in an increasingly AI-driven world.
Your next step:
If you’re navigating this transition and need guidance on validation frameworks, human oversight protocols, or TGA-aligned AI implementation—let’s talk. I build working systems with practising healthcare persons and QPPVs, not theoretical frameworks that sit on shelves.