Why We Do This
Because safety and trust aren’t automatic. AI is transforming how we identify signals, conduct audits, and make decisions—so we make it simple, ethical, and transparent. We combine human judgment with responsible AI to turn complexity into clarity, data into confidence, and obligations into action. In short: we protect patients, support professionals, and help innovation move forward—safely, together.
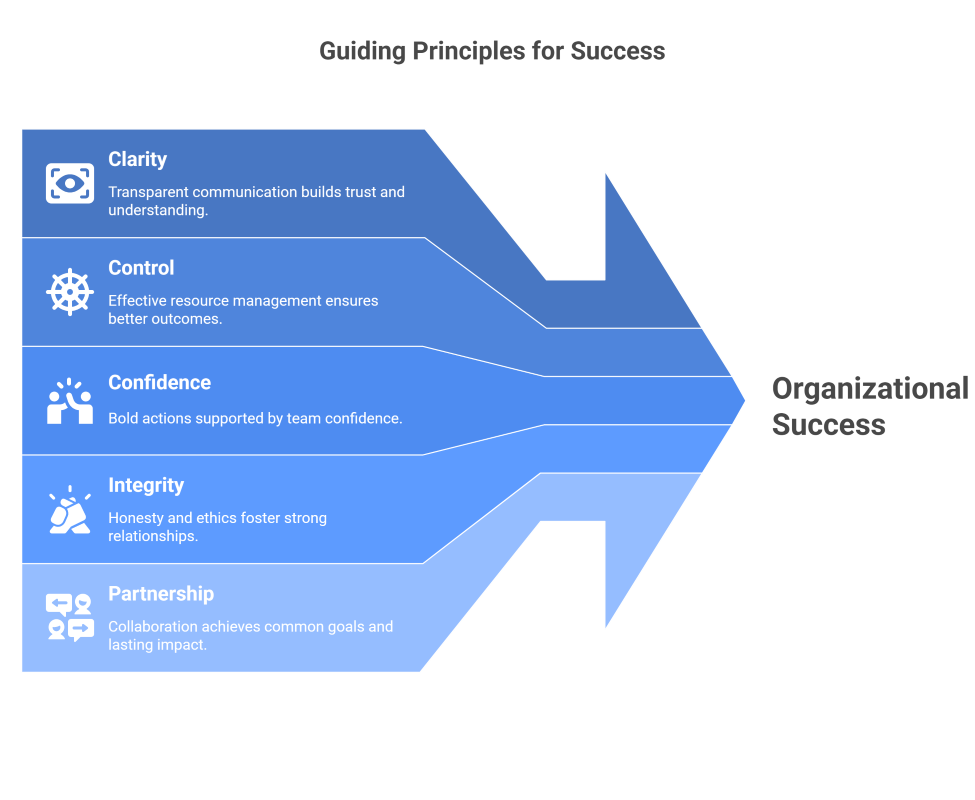
Every audit—and every compliance journey—starts with clarity.
GxPVigilance delivers independent PV, GCP, GLP, and ethics audits that strengthen systems, reinforce governance, and lift inspection readiness.
We design AI-enabled workflows and compliance tools that fit safely into daily operations, from automation agents and Microsoft Copilot to structured audit and risk solutions.
We also train teams to use AI responsibly for documentation, analysis, and decision support—while maintaining oversight, traceability, and quality.
Supporting Those Who Safeguard Others
We partner with healthcare professionals, sponsors, CROs, hospitals, and research institutions committed to patient care and ethical research.
Whether you need a GxP audit, a clinical trial review, or guidance on integrating AI responsibly, we work alongside your team—simplifying documentation, strengthening oversight, and improving inspection readiness.
From Regulatory Roots to AI Readiness
We believe compliance should enable progress—not restrict it.
Drawing on two decades of experience across pharmacy, clinical research, and regulation, we transform complex GxP obligations into clear, auditable, and intelligent systems that withstand inspection and drive improvement.
Our focus spans Pharmacovigilance (GVP), Good Clinical Practice (GCP), and Research Ethics. Because we understand Australian, New Zealand, and global frameworks, we help sponsors, CROs, labs, distributors, and ethics committees streamline processes and build confidence under inspection.
As part of the BioSyn-AI Group, we go beyond advisory and auditing. We deliver practical, AI-powered solutions that safely integrate AI into daily workflows—boosting consistency, speed, and transparency while protecting the integrity of your quality systems.
Our Core Services
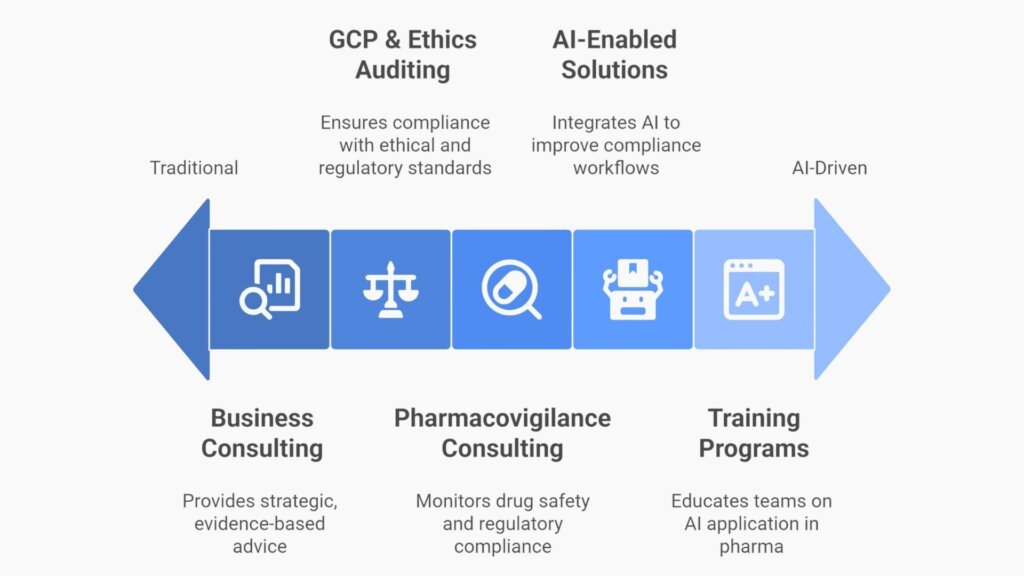
- Business Consulting & Strategic Advisory
We deliver evidence-based consulting for healthcare and life sciences—operational gap assessments, financial reviews, and executable strategies that strengthen governance and sustainability. - Pharmacovigilance Consulting & Auditing
We set up systems, provide QPPV/A-PVCP representation, oversee safety databases, monitor literature and signals, and drive inspection readiness with CAPA verification. - GCP & Ethics Auditing
We audit sponsors, CROs, sites, and ethics committees to ICH E6 (R3), NHMRC, and TGA expectations. - AI-Enabled Compliance Solutions in Pharma / Pharmacy
We integrate AI into regulated workflows to enhance traceability, document control, and reporting—while maintaining human oversight and complete audit trails. - Training & AI Readiness Programs
We deliver bespoke programs that help compliance, PV, and clinical teams understand, apply, and govern AI in real-world operations.
Our AI Solutions
AI Integration & Workflow Automation
We use Microsoft Copilot and leading LLMs to automate documentation, reporting, and auditing—reducing manual burden and improving consistency.Continuous AI Education & Regulatory Updates
We keep teams current with structured training, timely updates, and live insights.Tailored AI Governance & Compliance
We build governance frameworks, risk registers, and SOPs aligned with ISO 9001 and ISO 42001—ensuring traceability, transparency, and accountability.Enablement Roadmaps & Hands-On Training
We guide teams step-by-step to design, test, and deploy AI responsibly—always practical, auditable, and aligned with existing QMS and data-integrity principles.
Our Promise
We begin with clarity and finish with confidence.
We don’t just prepare organisations for inspections—we prepare them for the future.
Through compliance, technology, and partnership, we help regulated teams move forward with purpose, integrity, and intelligence.