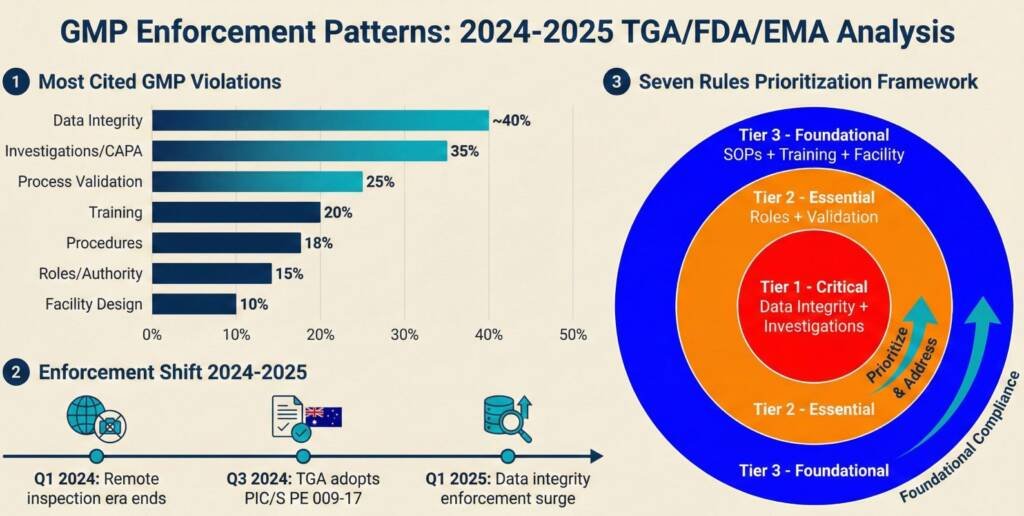
When the regulator arrives for a GMP audit, they assess one underlying question: Can this organisation consistently produce medicine that won’t harm anyone? Recent enforcement data reveal that seven specific GMP requirements account for approximately 70% of significant findings.
These aren’t esoteric technical standards. They’re foundational operational systems that regulators commonly assess every manufacturer to demonstrate from the first commercial batch. These areas reflect recurring inspection focus and enforcement trends, not a guarantee of inspection outcomes or a substitute for a complete pharmaceutical quality system
Australian pharmaceutical manufacturers face acute pressure in 2026. The TGA’s adoption of PIC/S Guide PE 009-17, effective September 2025, aligned Australia’s inspection expectations with international standards. Simultaneously, the regulator shifted from educational to enforcement by litigation, imposing substantial financial penalties. The medicinal cannabis and compounding sectors experienced this most dramatically, with companies receiving fines exceeding $300,000 for GMP non-compliance.
Rule 1: Design Facilities for Contamination Control From Day One
- Unidirectional flow prevents contamination backflow.
- Personnel flow control with physical gowning barriers
- Cleanroom classification supported by monitoring data
- HVAC pressure cascades with alarmed differentials
- Surface design with minimal dead spaces
Rule 2: Validate Every Process That Touches Product Quality
- Stage 1—Process Design: Define Critical Quality Attributes and Critical Process Parameters through development studies and risk assessment.
- Stage 2—Process Qualification: IQ/OQ/PQ protocols confirming equipment installation, operation, and commercial-scale performance.
- Stage 3—Continued Process Verification: Statistical process control during routine production, detecting drift before creating out-of-specification batches.
Rule 3: Write Procedures That Match Actual Practice
- Written at the executor competency level
- Tested by non-expert staff attempting to follow them
- Contain only necessary information.
- Include decision points with clear criteria.
- Reference but don’t repeat supporting documents.
Rule 4: Map Every Decision to a Named, Qualified Person
- Quality Unit Authority cannot be overridden by production or commercial functions.
- The Batch Release Authority requires a designated qualified person review before distribution approval.
- Deviation Classification specifies roles that determine severity.
- Change Control Approval requires quality unit review before validated system changes.
Rule 5: Build Data Integrity Into Every Record-Generating System
- Tower Laboratories (December 2025): FDA demanded retrospective review of all invalidated out-of-specification results, citing missing audit trails and shared credentials
- Valpharma International (EU, 2025): Statement of Non-Compliance for unauthorised IT system access allowing undetected data manipulation
- Glenmark (July 2025): Invalidating out-of-specification dissolution results without adequate investigation—retesting until obtaining passing results
- Electronic systems: Complete audit trails logging every data entry, modification, and deletion with user ID and timestamp
- Paper systems: Original data in indelible ink, corrections single-line struck through with reason and initials
- Hybrid systems: Electronic raw data retained with complete audit trails—paper printouts alone don’t satisfy expectations
Rule 6: Train Staff to Demonstrated Competency
- Initial GMP training covering pharmaceutical quality principles
- Job-specific training on role-relevant procedures
- Competency assessment through practical evaluation
- Periodic requalification annually or after role changes
- Training on deviations/changes when procedures change
- Documenting session attendance without verifying understanding
- Generic training does not address the specific procedures personnel perform.
- Allowing batch record signatures before demonstrating documented competency.
- Relying on “years of experience” without periodic requalification
Rule 7: Investigate Thoroughly—It’s Your First Defence
- Objective evidence supporting conclusions
- Systematic methodology (fishbone diagrams, 5-whys, fault tree analysis)
- Investigation depth proportional to failure severity and recurrence
- Multiple contributing factors are considered.
Prioritising GMP Improvements With Limited Resources
- Rule 5 (Data Integrity): Foundation for all other evidence
- Rule 7 (Investigations): Primary assessment of quality culture
- Rule 4 (Role Clarity): Prevents authority confusion
- Rule 2 (Validation): Proves process capability
- Rule 3 (Procedures): Must match practice
- Rule 6 (Training): Enables procedure execution
- Rule 1 (Facility Design): Limits what procedures can achieve
Conclusion
Common Questions and Answers
What is the difference between GMP and cGMP?
The “c” in cGMP stands for “current,” emphasizing that manufacturers must use up-to-date systems and technologies that align with the latest regulatory standards, rather than relying on outdated practices.
How do I ensure Data Integrity (ALCOA+)?
Maintain records that are Attributable, Legible, Contemporaneous, Original, and Accurate by implementing strong access controls, enabling electronic audit trails, and preventing unauthorized data changes through technical and procedural safeguards.
What are the requirements for Personnel Training and Qualification?
Training should be kept current and updated when processes, SOPs, or roles change. Competency should be assessed beyond attendance (e.g., practical demonstrations or knowledge checks), and temporary staff or consultants should meet the same role-based training expectations as full-time personnel.
How is “Validation” different from “Qualification”?
Qualification demonstrates that specific equipment or systems (e.g., HVAC) are fit for intended use through IQ/OQ/PQ, while validation provides documented evidence that an end-to-end process consistently produces product meeting predetermined specifications.
What should be included in a Quality Agreement for outsourcing?
Quality Agreements should clearly define responsibilities between the sponsor and CMO for change control, deviation management, investigations/CAPA, batch disposition, documentation, record retention, and audit rights to ensure ongoing compliance and accountability.
How do I handle “Out-of-Specification” (OOS) results?
OOS results require a structured scientific investigation, including hypothesis-driven root cause analysis and documented assessment of laboratory and manufacturing factors. Retesting must be justified and controlled, and batch release decisions must be based on the full investigation, not on a passing retest alone.
What are the expectations for Cleaning Validation?
Cleaning validation should demonstrate that residues and contaminants are consistently removed to prevent cross-contamination. This includes selecting appropriate residues to test for, defining acceptance limits, and establishing worst-case scenarios based on factors like toxicity, solubility, equipment design, and product carryover risk.
How often should a Product Quality Review (PQR) be conducted?
PQRs are typically performed annually, with trending of critical quality data over time. For low-frequency products, organizations should define an approach that still evaluates product and process performance, potentially leveraging multi-year data to support meaningful trend analysis.
What is the correct procedure for Change Control?
Change control should assess potential impact to product quality, validation status, regulatory commitments, and supply continuity before implementation. This includes risk assessment, required testing or requalification, stakeholder approvals, implementation planning, and effectiveness checks after the change is executed.
Reference:
- Facts About the Current Good Manufacturing Practice (CGMP) – Link
- Warning Letter – Link
- FDA: Guidance for Industry – Process Validation: General Principles and Practices – Link
- Determination of the cross-contamination and validation of the cleaning process for an automated personalised dosing system – Link
- How to Evaluate and Demonstrate the Effectiveness of a Pharmaceutical Quality System in relation to Risk-based Change Management – Link
- TGA – Good manufacturing practice (GMP) – Link
Disclaimer
This article is provided for educational and informational purposes only. It is intended to support general understanding of regulatory concepts and good practice and does not constitute legal, regulatory, or professional advice.
Regulatory requirements, inspection expectations, and system obligations may vary based on jurisdiction, study design, technology, and organisational context. As such, the information presented here should not be relied upon as a substitute for project-specific assessment, validation, or regulatory decision-making.
For guidance tailored to your organisation, systems, or clinical programme, we recommend speaking directly with us or engaging another suitably qualified subject matter expert (SME) to assess your specific needs and risk profile.



