Animal Ethics External Review
When Your AEC System Needs Independent Oversight
I don’t show up, check boxes on a template, write generic findings, and invoice. I audit your AEC operations alongside your team, document what actually matters for the Code and your governing body, help you close critical gaps, and equip you to defend your systems effectively.
When Teams Work With Me
- Your four-year independent external review is due — need an auditor who understands both the Code requirements and the operational reality of running research programs.
- You’re preparing for a state/territory inspection — want someone who knows what regulators actually look for and can get you ready.
- Your AEC keeps repeating the same issues — protocol delays, incomplete documentation, unclear delegations, or ineffective monitoring — and you need an objective assessment of what’s broken.
- Complaints or non-compliance incidents have exposed system weaknesses — need an independent review that your governing body can trust.
- Your institution is expanding research operations — scaling from small programs to multi-site complexity, and your AEC governance needs to keep pace.
- You’re unsure if your AEC composition or processes actually meet the Code — the categories (A-D), independence requirements, Terms of Reference, and decision-making frameworks all feel uncertain.
What Makes This Review Different
Implementation Focus, Not Assessment-Only
Australian Code Context
ISO-Aligned Audit Methodology
Capability Transfer Embedded Throughout
Review Scope — What Gets Assessed
Governance & Independence: AEC composition (A–D), independence checks, ToR adequacy, quorum, COI control, decision process, chair support.
Institutional Responsibilities: Resourcing, Code-aligned policies, 3Rs promotion, leadership accountability, accessible procedures, governance reporting.
Protocol Lifecycle & Oversight: Ethical review and approval, monitoring, AE management, amendments control, project closure verification.
Facilities & Animal Care: Housing standards, husbandry, veterinary oversight, humane endpoints, staff competence, records, breeding oversight.
Complaints & Non-Compliance: Intake/triage, investigations, RCA effectiveness, CAPA implementation/verification, trends, lessons learned.
Transparency & Improvement: Use prior reviews, annual reports, appropriate public transparency, evidence of system maturation.
How The Review Works
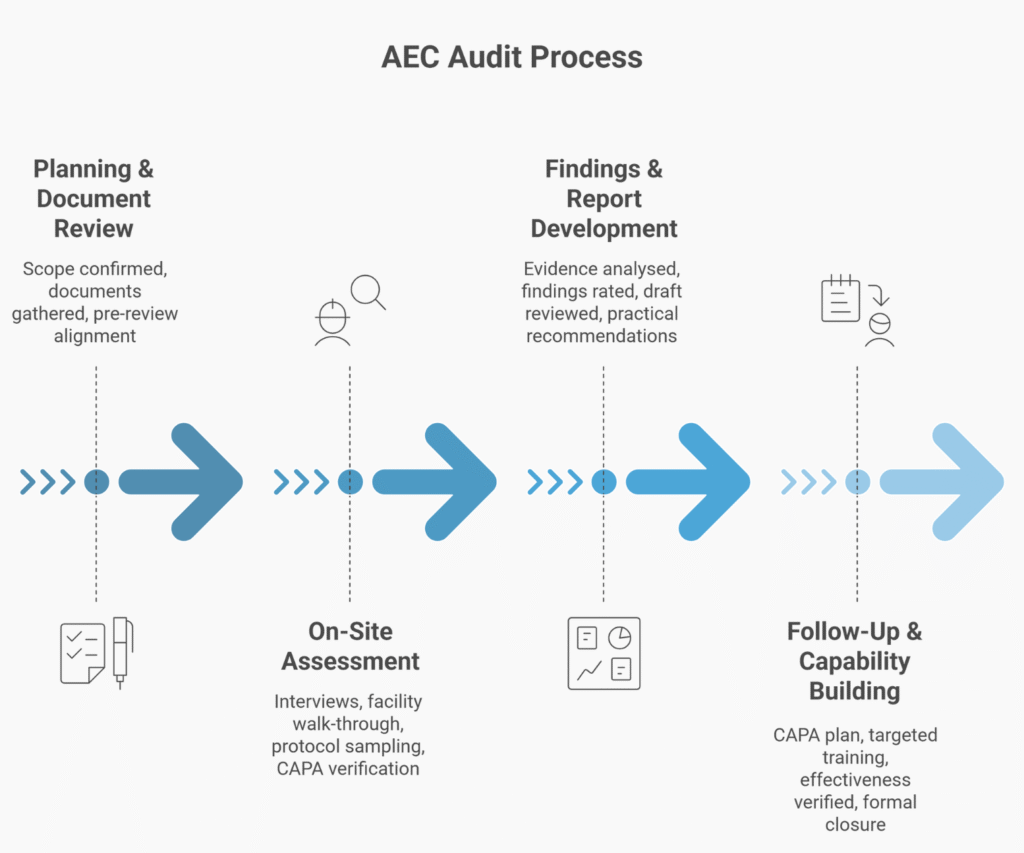
What You Receive
- Independent External Review Report — clear, defensible report mapping findings to the Code and state/territory requirements, with an overall assurance statement for your governing body.
- Executive Summary — concise brief highlighting key strengths, critical findings, and priority actions for leadership decision-making.
- Risk & Maturity Profile — assessment of system strengths and vulnerabilities against Code expectations, with prioritised improvement roadmap.
- CAPA Recommendations (Where agreed) — practical, proportionate recommendations with regulatory references and implementation guidance. You set owners and timelines; I verify effectiveness.
- Supporting Evidence Package (Where agreed) — audit trail documentation, interview notes, observation records, and sampled files that demonstrate review rigour and support conclusions.
Who This Serves
- Universities and research institutes conducting animal research must comply with the Code.
- Hospitals and health services with animal research programs or shared AEC arrangements.
- Biotechnology companies and CROs are establishing or maintaining AEC governance.
- Multi-site organisations needing consistent standards across locations
- AEC secretariats seeking a credible, independent assessment of effectiveness
- Institutions preparing for state/territory audits or NHMRC funding assurance
Why GxPVigilance
- Independence You Can Defend
Review and audit services only (no operational delivery), ensuring objectivity and avoiding conflicts. Findings that your governing body and regulators can trust. - AEC-Specific Expertise
Direct experience in ethics committees, research governance, and Australian healthcare operations. I understand how AECs actually function — not just how they’re supposed to work on paper. - ISO-Aligned Quality
Every engagement is conducted under our QMS. Audit trails, risk-based sampling, and proportionate findings that withstand regulatory scrutiny. - Values That Match Your Mission
Clarity. Control. Confidence. Integrity. Authenticity. Progress through partnership, not perfection.



