Inspection-Ready. Patient-Centred. Compliance with Confidence.
At GxP Vigilance, we deliver GVP (Good Pharmacovigilance Practice) consulting and auditing services across Australia and into New Zealand because we believe ensuring patient safety and regulatory compliance is a journey we take together.We partner with sponsors, distributors, and pharmacovigilance service providers to strengthen your post-market safety systems, so you meet regulatory expectations with integrity and assurance.
What Makes Us Different
Implementation Focus, Not Assessment-Only
Most auditors show up, find gaps, write 40 pages of findings categorised by minor, major or critical, and leave. You’re left with a report, recommendations, and the same overwhelmed team expected to fix everything. We don’t work that way for all clients! We audit your pharmacovigilance system alongside your team, document what matters, help you close critical gaps during the engagement, and equip you to defend your decisions when the TGA inspector arrives. You get working systems and capability transfer—not consultant dependency.
Australian TGA Context Expertise
Deep familiarity with TGA’s Pharmacovigilance Responsibilities of Medicine Sponsors guidance, local reporting timelines, QPPV/A-PVCP requirements, and what Australian inspectors actually look for during GVP assessments.
We are not translating generic international frameworks. We help develop systems that operate within your regulatory environment—systems that align with Australian healthcare realities while remaining internationally compliant where necessary.
Capability Transfer, Not Dependency
Every audit includes embedded training. If appropriate, your team learns why findings matter, how to write effective CAPAs, how to demonstrate compliance under questioning, and how to maintain the system after we leave.
You own the capability when we’re done—not just a list of corrective actions.
Patient Safety Non-Negotiable
Every finding, every recommendation, every system improvement anchors back to patient safety. Fast and safe. Efficient and compliant. Never one without the other.
Why GVP Auditing Matters
Regulators such as the Therapeutic Goods Administration (TGA) in Australia expect sponsors of medicines to maintain a robust pharmacovigilance system. By conducting a well-structured GVP audit, you will:
- Verify that adverse events and safety data are collected, assessed, and reported in line with required timelines.
- Confirm that case-management and signal-detection procedures are traceable, effective, and risk-proportionate.
- Ensure governance documents (SOPs, risk-management plans, signal trackers) remain current, auditable, and aligned with your obligations.
- Define local roles clearly (such as the Qualified Person for Pharmacovigilance in Australia) and support them with documented processes.
We believe that compliance is not just about passing an inspection — it is about building trust through transparency, consistency, and readiness.
Comprehensive GVP Audit Scope
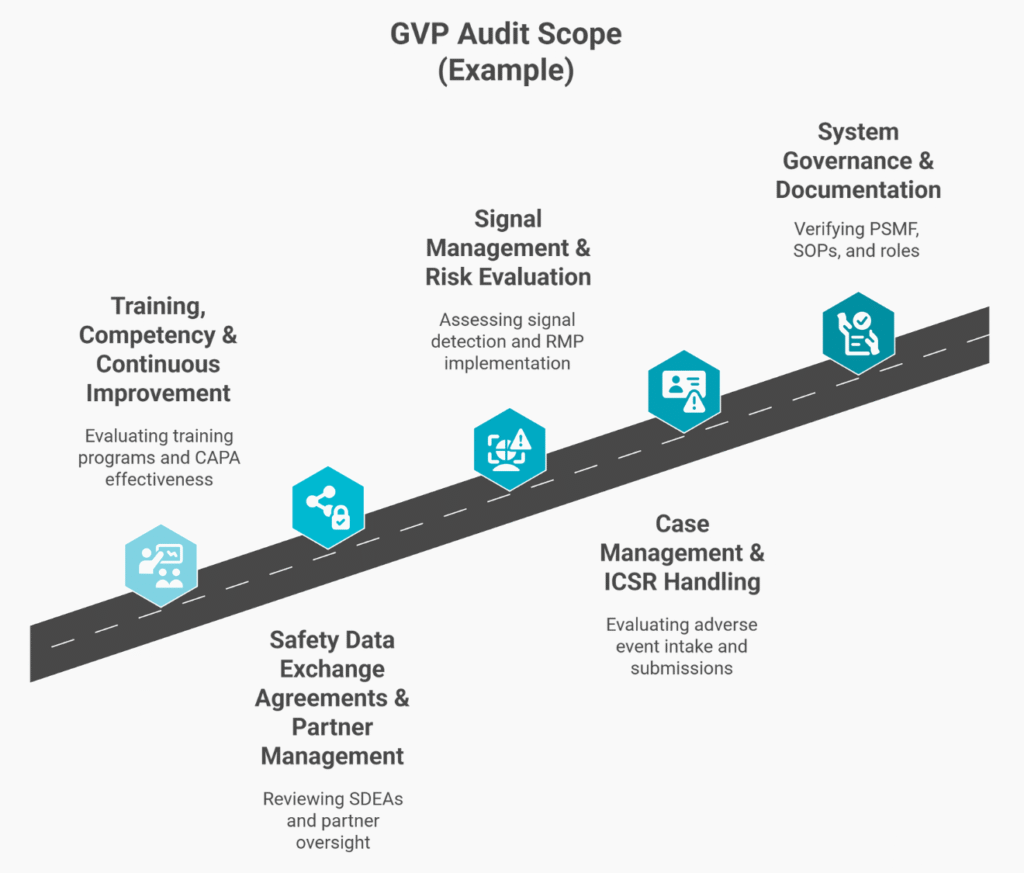
Our Approach
- Phase 1 — Initiation & Planning: Define purpose, scope, criteria, resources, and responsibilities; align objectives and timelines.
- Phase 2 — Engagement & Preparation: Confirm authority, communications, confidentiality, and logistics; draft a risk-based audit plan and checklists.
- Phase 3 — Document & Evidence Review: Collect and screen policies, procedures, and records to understand the system design and controls.
- Phase 4 — Audit Execution (Virtual/On-Site): Conduct interviews, observe, and collect samples; verify implementation against criteria with objective, traceable evidence.
- Phase 5 — Evidence Evaluation & Findings: Analyse conformity, classify nonconformities by risk/impact, and substantiate with objective evidence.
- Phase 6 — Reporting & Communication: Deliver a clear report of scope, evidence, findings, and conclusions; agree on corrective action priorities.
- Phase 7 — Follow-Up & Closure: Verify CAPA implementation and effectiveness; capture lessons learned and formally close the audit.
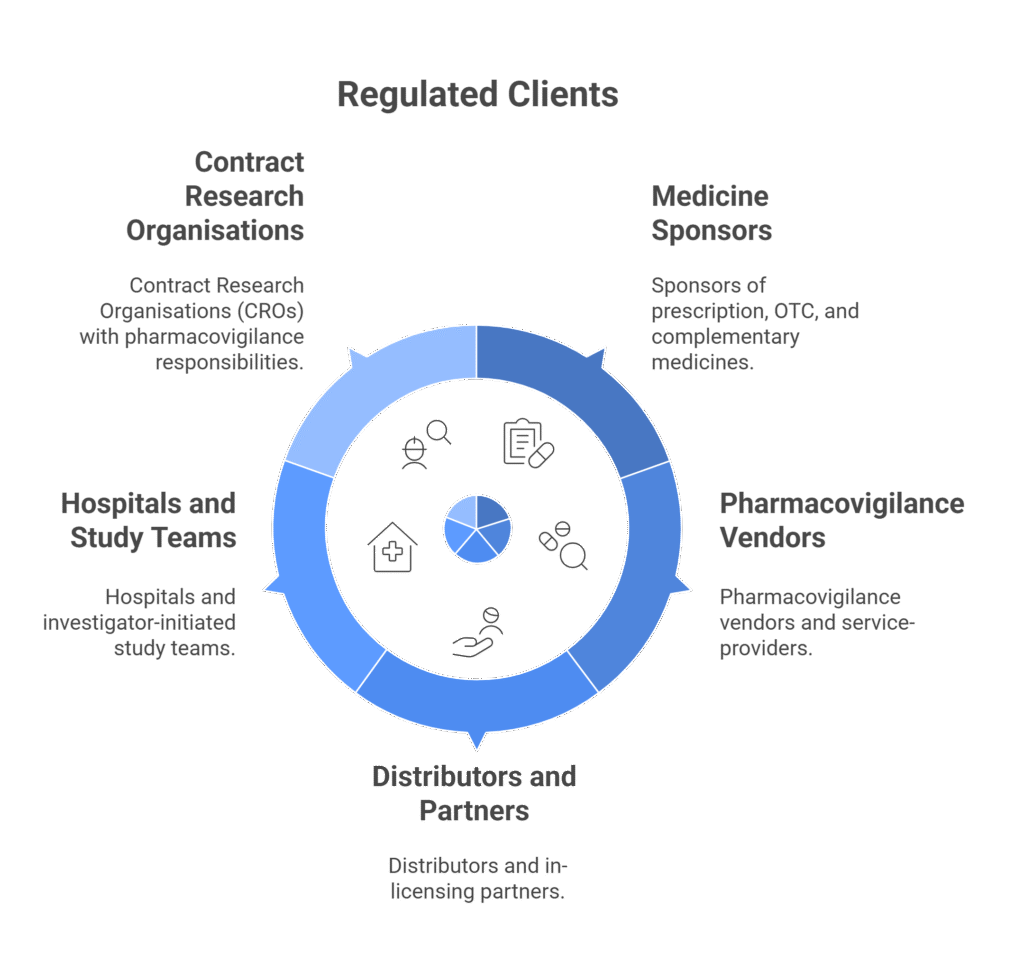
Who We Serve
Why Choose GxP Vigilance
- Experienced Auditors: Our auditors hold decades of expertise in pharmacovigilance and regulatory affairs — they know what regulators expect, and they guide you accordingly.
- Local Expertise: We understand the TGA and New Zealand regulatory frameworks — and we align your systems with global best practice (including EU GVP guidance adopted in Australia).
- End-to-End Oversight: From planning through to CAPA closure and follow-up, we support you every step of the way.
- Human + AI Insight (Optional): For clients who adopt advanced tools, we incorporate AI-assisted analytics to enhance case tracking, literature review, and signal trend detection.
Your Next Step
If your PV system feels unsustainable, if you’re preparing for TGA inspection, or if you need more than another audit report—let’s have a conversation.
Contact us to discuss where pressure is highest, what inspection readiness actually requires, and whether this approach aligns with your team’s needs.



