When Clinical Operations Overwhelm Your Team
You’re preparing for an audit, inspection, or want to ensure you’re ready for the next phase of your organisation’s growth, but you’ve identified gaps and aren’t sure where to start. Protocol deviations keep happening despite training. QMS isn’t operational. RBQM feels like compliance theatre. Your team is aware of the issues, but they can’t find the time to fix them while trials are running.
We’re the team you call when you need inspection-ready clinical operations—not just an audit report.
We don’t show up, find gaps, write 40 pages of findings, and disappear. We audit your GCP compliance alongside your team, document everything that matters, help you close critical gaps, and equip you to defend your systems under TGA scrutiny.
Clinical research in Australia relies on robust oversight, ethical conduct, and transparent evidence—principles mandated by the Therapeutic Goods Administration (TGA), the National Health and Medical Research Council (NHMRC), and ICH GCP E6 (R3). Sponsors, CROs, investigator sites, research institutions, and hospital teams must demonstrate not just compliance but also traceable, risk-managed operations and ethical participant protection.
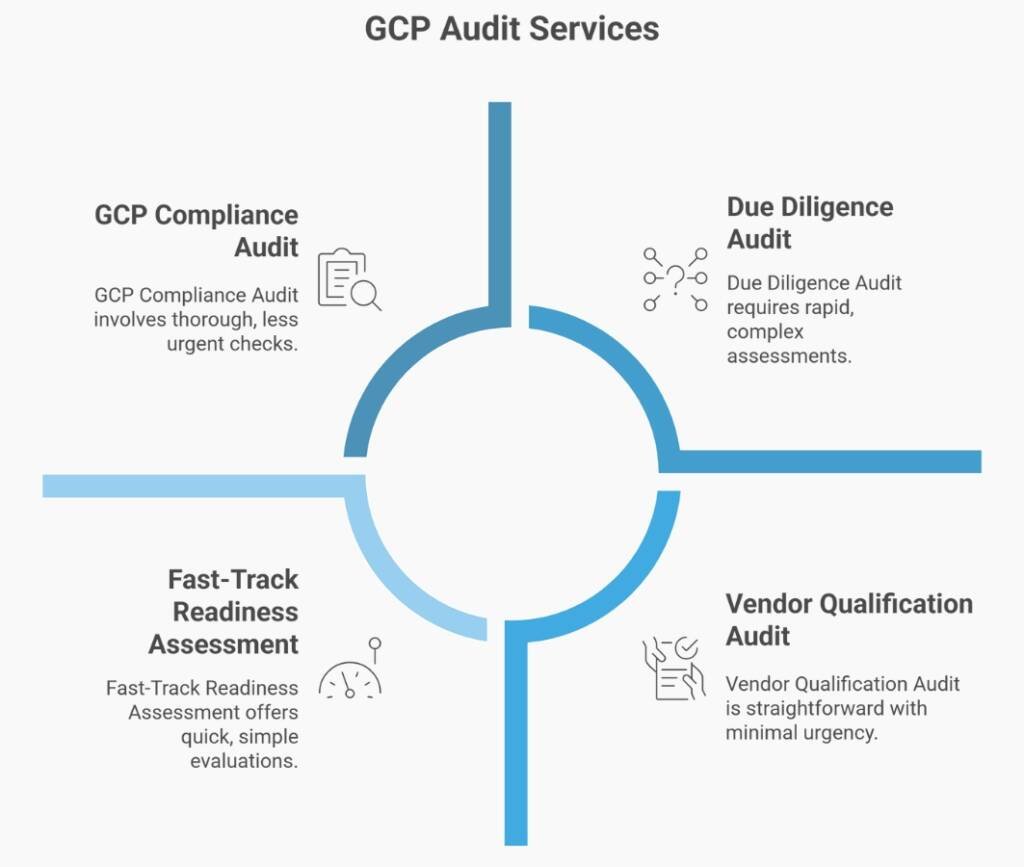
For International Organisations: Vendor qualification Audits in Australia
When You Need Ground Truth Fast
You’re a US/European/Asian sponsor evaluating Australian sites for trial participation. You’re a CRO assessing vendor capabilities before contract signature. You’re an investor conducting pre-acquisition due diligence on an Australian clinical research organisation. You need an independent, credible assessment of operational capability—and you need it in weeks, not months.
I provide rapid, commercially-focused due diligence audits that answer the questions keeping your deal on hold.
What Makes Our Due Diligence Different
- We understand both sides of the equation. I speak your language (ICH, FDA, EMA standards) and Australian regulatory reality (TGA, HREC, local operational constraints). You get assessment through both lenses—what the site claims versus what they can actually deliver under Australian conditions.
- Speed without shortcuts. We mobilise within days, conduct on-site assessment within 1-2 weeks, and deliver initial findings within 48 hours of completion. Full report with risk-rated findings, remediation roadmap, and go/no-go recommendation within 5 business days.
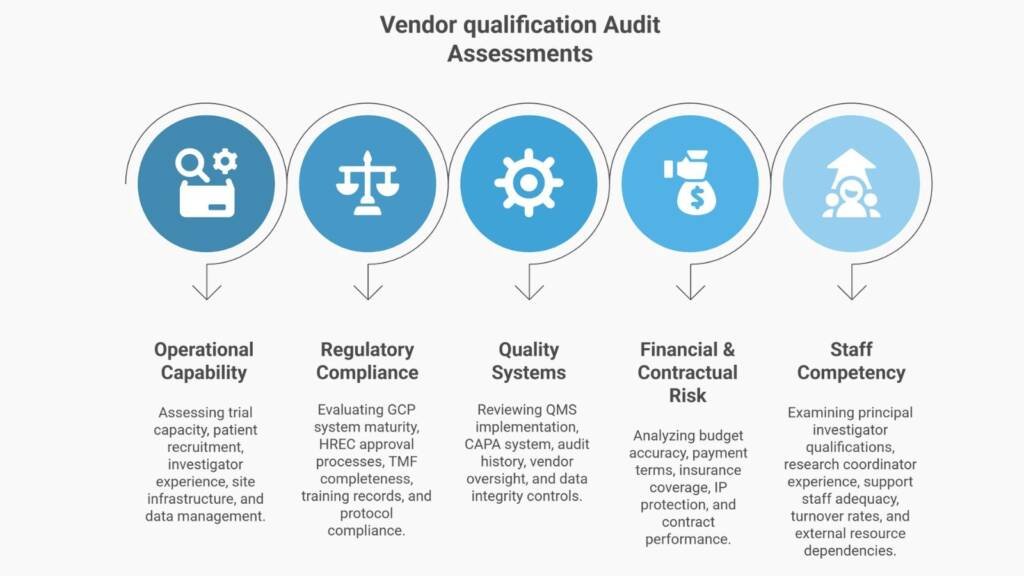
- Commercial clarity, not just compliance. Due diligence isn’t only about regulatory gaps. It’s about operational capability, financial viability, staff competency, system scalability, and contractual risk. We assess all of it and tell you what matters for your decision timeline.
- Australian regulatory expertise. For overseas organisations unfamiliar with TGA requirements, HREC approval processes, or Australian clinical trial conduct, we translate local regulatory expectations into practical operational implications. You’ll understand what compliance actually requires in this market.
- Independence you can defend. No operational conflicts. No consulting relationships with audited organisations. Objective findings that your stakeholders can trust when making investment or partnership decisions.
For Vendors & Sites Being Audited
A Partnership, Not an Interrogation
If you’ve been selected for vendor qualification or a routine oversight audit, here’s what to expect:
- We work with you, not against you. I have managed clinical trial supply chains and navigated operational challenges while ensuring compliance. I have sat on ethics and clinical trial protocol committees, been involved in regulatory inspections, and work daily with colleagues in this field. I understand real-world constraints, including staff shortages, system limitations, and competing priorities. My role is to help you demonstrate capability, identify areas for improvement, and build confidence with your sponsor or regulatory authority.
- Clear communication throughout. Pre-audit briefing explains the scope, timeline, and what we’ll review. During the audit, findings are discussed in real-time, ensuring no surprises in the final report. The post-audit debrief focuses on constructive improvement, rather than blame.
- Australian regulatory context explained. For overseas vendors new to TGA requirements or Australian sponsors, I translate what ICH E6(R3), TGA GCP expectations, and local HREC standards actually mean in practice. You’ll understand not just what’s required, but why it matters and how to demonstrate compliance.
- Findings that improve capability. Every finding includes objective evidence, regulatory reference, risk rating, and practical remediation guidance. The goal is to strengthen your systems, not create a paperwork burden.
- Professional respect. You’re experts in your domain. I’m here to assess against regulatory standards, help you close gaps, and ensure your quality systems are ready for inspection. That requires partnership.
What Makes This Different
- Implementation Focus, Not Assessment-Only: Most auditors identify problems and leave. I help you fix them. Critical gaps get closed during the engagement, not months later when the inspection notice arrives.
- Australian TGA Context (Explained Clearly): Deep familiarity with TGA GCP inspection themes, local HREC requirements, and the Australian clinical trial environment. For overseas vendors and international due diligence clients, I translate Australian regulatory expectations into practical operational requirements—no assumptions about prior TGA knowledge.
- Commercial Realism for Due Diligence: I understand that business decisions have timelines. Due diligence reports answer: “Can they deliver?” “What are the risks?” “Should we proceed?” You get clear recommendations, not just data.
- Capability Transfer: Your team learns why findings matter, how to write effective CAPAs, and how to defend decisions under inspection questioning. Training is embedded throughout.
- Operational Realism: I’ve managed hospital pharmacy operations, dealt with staff constraints, and worked within system limitations. Findings acknowledge real-world context while maintaining regulatory standards.
- Inspection-Ready by Design: Everything documented to withstand TGA scrutiny. Audit trails, risk assessments, oversight evidence—prepared for the questions inspectors actually ask.
- Independence Guaranteed: I provide audit services only (no operational delivery), ensuring objective and defensible findings you can trust.
Auditor Credentials and Independence
GCP Vigilance’s lead auditors bring 8 years of hands-on experience across Australia’s clinical trial, medicine safety and quality management system ecosystem, including CROs, research institutions, universities, and hospitals. Our credentials include:
ISO 9001, ISO/IEC 17025, ISO 13485 auditor credentials, ensuring methodical execution, technical depth for laboratories and device studies
Computerised System Validation (CSV) expertise for assessing eTMF, EDC, CTMS, and LIMS systems—critical for TGA data integrity focus
ISO 42001 (AI management systems) training positions us uniquely to assess AI-enabled trial systems as Australia’s regulatory landscape evolves
Independence guarantee: We provide only audit services (no operational delivery), ensuring objective, defensible assurance
Experience outside auditing: Pharmacy, Ethics Committee, CSV, Clinical Trial Protocol and early phase regulatory affairs and compounding.
Frequently asked questions
GCP auditing verifies that clinical trials are conducted according to Australian regulatory requirements, ethical standards, and ICH Good Clinical Practice. It helps sponsors and investigators demonstrate oversight, protect participants, and ensure data integrity. In Australia, TGA and NHMRC guidance make this oversight a core expectation for all clinical research sponsors.
Our audit checklists, interviews, and reporting structure mirror the TGA’s inspection process. We incorporate current inspection focus areas and metrics, so your systems, documentation, and CAPAs are aligned with what TGA inspectors actually test during real inspections.
Australian GCP audits emphasise ethical oversight, local sponsor accountability, and compliance with NHMRC safety monitoring and reporting. We ensure documentation reflects local expectations, including ethics committee communication and CTN-specific sponsor responsibilities.
ICH E6 (R3) introduces stronger requirements for risk-based quality management (RBQM), critical-to-quality (CTQ) factors, and data traceability. Our audits operationalise these concepts in practical terms — checking that controls, evidence trails, and vendor oversight are risk-proportionate and documented clearly.
Our clients include trial sponsors, CTN holders, CROs, investigator sites, and hospital-led research units. We also audit laboratories, vendors, and ethics committees involved in clinical research oversight. Essentially, any organisation with trial responsibilities under Australian GCP may benefit from an independent audit.
We follow a structured, ISO-aligned methodology: pre-audit planning, evidence review, on-site or remote audit execution, and CAPA follow-up. Each step is traceable, transparent, and delivered in partnership with your team to ensure compliance and readiness without unnecessary disruption.
No. We maintain independence by focusing solely on auditing and CAPA follow-up support. We do not perform GCP operations ourselves, ensuring our findings remain objective and free from conflict of interest.