Every prescription medicine and biological registered in Australia carries ongoing obligations that extend well beyond approval. Among the most significant is the Risk Management Plan, a document the Therapeutic Goods Administration uses to verify that sponsors have genuinely thought through how their product’s safety profile will be monitored and managed throughout its commercial life. For sponsors operating across multiple jurisdictions, the Australian approach presents a particular challenge: adapting a document developed for European regulators to a different healthcare system, epidemiology, and patient population.
This is the Australian story of Risk Management Plans—not the theory, but the practical decisions sponsors must navigate when building an RMP that satisfies TGA expectations while remaining proportionate to actual risk.
What Makes the Australian RMP Submission Different?
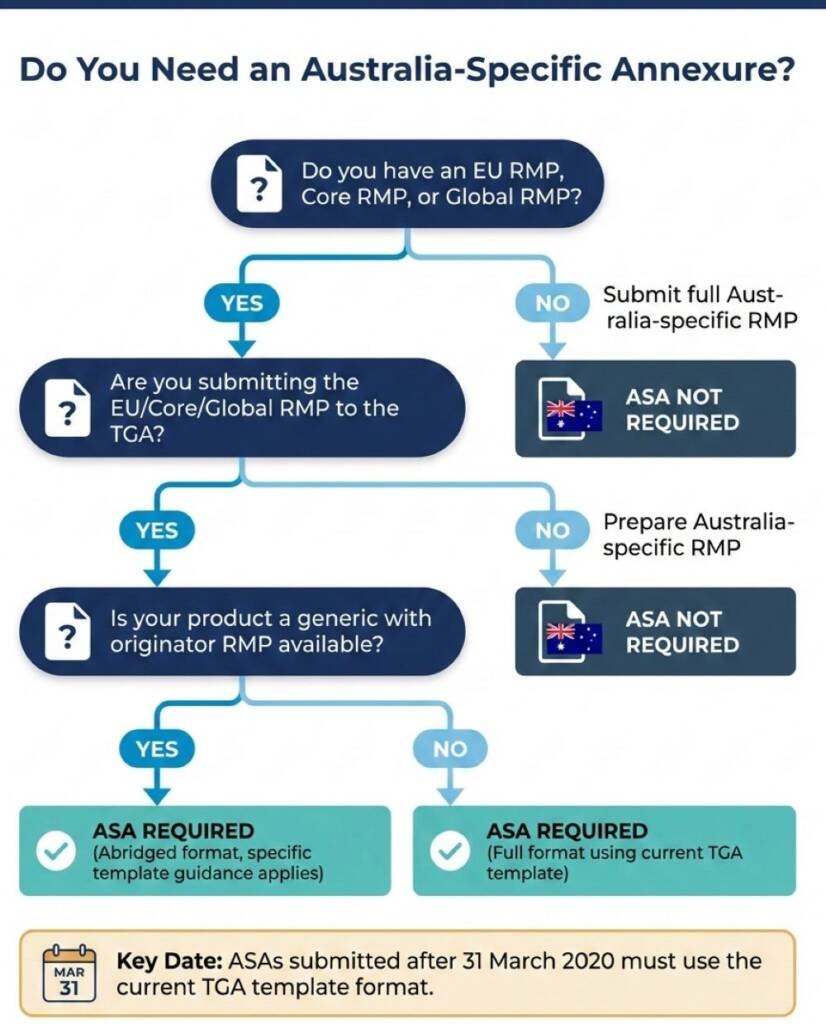
The TGA adopts EU Good Pharmacovigilance Practice guidelines, including GVP Module V on risk management systems. However, adoption does not mean identical implementation. When sponsors submit Risk Management Plans to the TGA, they typically provide two documents working together: the EU RMP itself, plus an Australia-Specific Annexure that documents differences between European and Australian circumstances.
The ASA exists because risk is context-dependent. A safety concern prominent in one population may be less relevant in another. Risk minimisation measures implemented through European healthcare structures may require adaptation to Australian dispensing practices, prescriber behaviours, or patient access pathways. The TGA expects sponsors to demonstrate they have considered these differences rather than submitting EU documentation unchanged.
| Scenario | RMP required | ASA required |
|---|---|---|
New chemical entity with EU RMP | Yes | Yes |
New biological with EU RMP — plus biologic-specific sections | Yes | Yes |
Generic with originator RMP available Abridged RMP acceptable | Yes | Yes — using specific template guidance |
No EU, core, or global RMP exists Australia-specific RMP | Yes | No — full Australian RMP substitutes |
Product already on ARTG, safety concern identified | Yes, if requested | Yes |
The only situation where an ASA is not routinely required is when no EU, core, or global RMP exists, and the sponsor submits a full Australia-specific RMP instead.
How Should Sponsors Approach the ASA?
The TGA provides a template for the Australian Specific Annexure, and submissions after 31 March 2020 must follow the current format. The template is not simply a checklist—it requires substantive analysis across four domains.
Product overview and registration context. Sponsors document the product’s regulatory history, including approval dates, ARTG numbers, and any previous safety-related variations. This section establishes baseline context for evaluators.
Safety specification alignment. This is where the analytical work happens. Sponsors must compare the summary of safety concerns from the EU RMP against Australian epidemiological data and identify whether differences exist. If Australian disease prevalence differs from Europe, or if risk factors distribute differently across the population, these differences require explicit discussion. The TGA does not expect sponsors to generate new Australian data, but it does expect reasoned analysis of whether existing safety concerns apply equally, less, or more in Australia.
Pharmacovigilance plan localisation. Routine pharmacovigilance activities described in the EU RMP need to be translated for Australian operations. Sponsors explain how adverse event collection, signal detection, and ongoing benefit-risk monitoring will function within Australian systems. For biologicals, additional biovigilance requirements apply.
Risk minimisation measure adaptation. Perhaps the most consequential section. If the EU RMP includes additional risk minimisation measures—educational programmes, controlled access, prescriber restrictions—the ASA must describe how these translate to Australian practice. Sometimes a European measure has no Australian equivalent. Sometimes, Australian healthcare structures enable different approaches. The sponsor’s job is to demonstrate that Australian patients receive equivalent protection through context-appropriate means.
What Does the TGA Actually Evaluate?
When TGA evaluators review Risk Management Plans, they assess whether the documented system adequately addresses the product’s important risks. Their focus is practical: will this RMP, if implemented as written, provide adequate safety monitoring and appropriate risk control for Australian patients?
Evaluators look for evidence of genuine local consideration rather than generic adaptation. Common evaluation concerns include:
- Unchanged safety specifications that fail to address Australian epidemiological differences
- Risk minimisation measures referencing European infrastructure that does not exist in Australia
- Missing justifications for why EU pharmacovigilance activities remain relevant despite different healthcare contexts
- Biovigilance gaps for biological products, where traceability and immunogenicity monitoring requirements differ
The evaluation sits within the broader registration or inclusion process. For prescription medicines, RMP evaluation integrates with clinical and non-clinical assessment at defined milestones. The RMP evaluator’s recommendations feed into the conditions of registration.
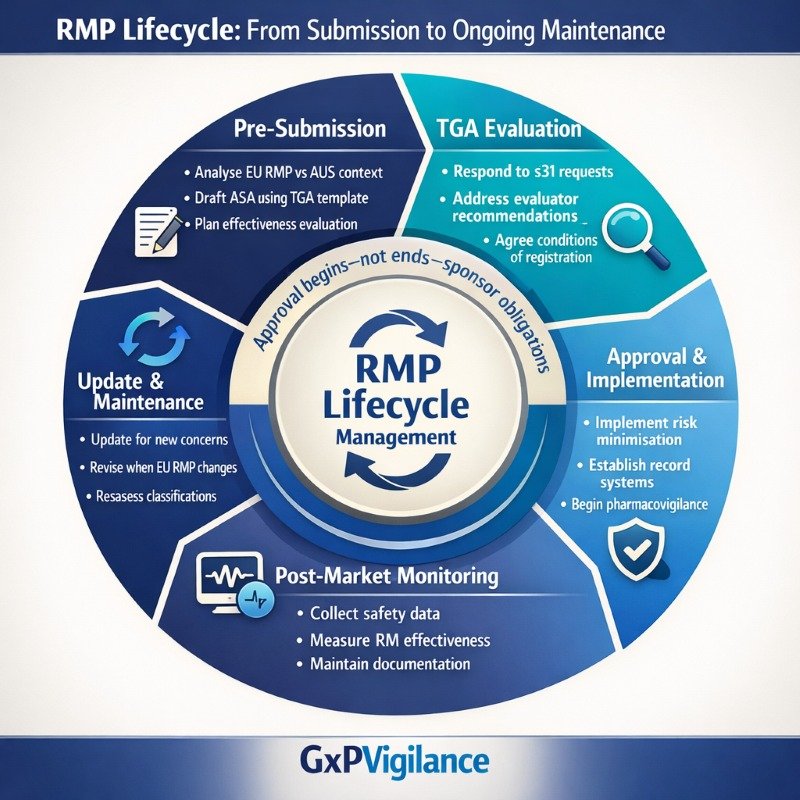
The Lifecycle Obligation: RMPs Are Living Documents
Approval of a Risk Management Plan does not end sponsor obligations—it begins the lifecycle management phase. The TGA expects sponsors to maintain their RMPs as living documents that evolve as safety knowledge accumulates.
Mandatory update triggers include:
- New safety concerns identified through signal detection
- Significant changes to risk minimisation measures
- Requests from the TGA based on emerging safety data
- Changes to the EU RMP that affect Australian implementation
- Modifications to Product Information affecting safety messaging
Sponsors should not wait for TGA requests before updating. Proactive maintenance demonstrates good pharmacovigilance practice and avoids situations where RMP documentation lags behind actual safety knowledge.
Common lifecycle maintenance gaps:
✓ Failing to update the ASA when EU RMP revisions occur ✓ Not documenting Australian-specific signal evaluation ✓ Continuing risk minimisation measures past their useful life ✓ Missing records of effectiveness evaluation
Aligning with EMA’s Risk-Proportionate Approach
Understanding the EU foundation helps sponsors build better Australian submissions. GVP Module V shifted EMA expectations toward focused, actionable, and risk-proportionate Risk Management Plans. The revision encourages sponsors to include only those safety concerns genuinely requiring ongoing characterisation or specific risk minimisation.
This principle directly translates into Australian practice. The TGA does not expect inflated safety specifications that list every possible concern regardless of materiality. Instead, evaluators look for thoughtful prioritisation—safety concerns selected because they matter for Australian patients, with proportionate activities designed to address them.
The recent EMA GVP Module XVI Revision 3 (effective August 2024) updates guidance on risk minimisation measures, including new requirements for evaluating effectiveness. Australian sponsors referencing EU measures should verify that their ASAs reflect current EMA expectations, as the TGA adopts these guidelines.
Practical Decisions Sponsors Must Make
Risk Management Plans require sponsors to make consequential choices, not merely complete templates. The decisions that matter most:
Which safety concerns warrant Australian-specific attention? Not all EU concerns require identical treatment. Some may be more relevant in Australia due to local prescribing patterns or population characteristics. Others may be less relevant. The sponsor’s analysis should be explicit.
What evidence demonstrates adequate risk control? Process indicators (e.g., was the educational material distributed?) differ from outcome indicators (e.g., did prescribing behaviour change?). The TGA increasingly expects sponsors to plan for effectiveness evaluation from the outset.
How will you demonstrate implementation? RMP commitments become conditions of registration. Sponsors must maintain records proving they fulfilled their obligations—distribution logs, training completion records, signal evaluation documentation, and effectiveness study results.
When will you revisit safety concerns? GVP Module V explicitly enables removal or reclassification of safety concerns when evidence supports such changes. Sponsors should plan periodic reassessment rather than treating initial safety specifications as permanent.
Inspection and Audit Considerations
TGA pharmacovigilance inspections assess whether sponsors comply with their documented obligations. For Risk Management Plans, inspectors verify that commitments made in the RMP-ASA are translated into operational reality. Inspection findings have included:
- Failure to produce records verifying fulfilment of post-approval commitments
- Failure to notify the TGA regarding intended significant changes to the RMP-ASA before implementation
- Inadequate documentation of the risk minimisation measure effectiveness evaluation
These findings highlight the gap between documented plans and operational execution—a gap that well-prepared sponsors close through systematic record-keeping and proactive maintenance.
Building RMPs That Work
Effective Risk Management Plans share common characteristics: they address genuine safety concerns proportionate to the product’s risk profile, they translate meaningfully to the Australian healthcare context, and they create monitoring systems the sponsor can actually implement and document.
The Australian story of Risk Management Plans is ultimately about local accountability. Sponsors cannot simply export European documentation and expect TGA acceptance. The ASA requirement forces genuine consideration of Australian circumstances—and that consideration protects Australian patients.
For sponsors preparing submissions, the practical path forward involves: accessing current TGA guidance and templates, analysing EU RMP content against Australian epidemiology and healthcare structures, documenting local adaptations with a clear rationale, and planning lifecycle maintenance from day one. The effort invested in building a thoughtful RMP pays returns throughout the product’s commercial life in Australia.
Common Questions and Answers
Q1 – When must I submit a Risk Management Plan to the TGA?
The TGA requires RMPs to be submitted with certain higher risk applications to enter a prescription medicine or biological in the ARTG, or to vary an ARTG entry where there are significant safety implications. The TGA can also request an RMP (or RMP update) for products already on the ARTG if new safety concerns emerge or if existing risk minimisation is no longer considered adequate.
Q2 – Can I submit an EU RMP without an Australian Specific Annexure?
You may submit an Australia specific RMP without an ASA only when no EU, core or global RMP exists and you prepare a full Australia specific RMP instead. In all other situations—when you submit an EU RMP or a core/global RMP—the TGA expects an accompanying Australian specific annex (ASA) documenting Australian specific safety concerns, pharmacovigilance activities and risk minimisation.
Q3 – What template should I use for the ASA?
The TGA provides a standard ASA template and expects ASAs submitted with contemporary applications to follow this format. Earlier ASAs prepared before the template was introduced may use older formats, but new or updated ASAs should follow the current template and guidance notes to support efficient evaluation.
Q4 – How do I determine whether Australian safety concerns differ from the EU RMP?
To determine whether Australian safety concerns differ from those in the EU RMP, compare Australian epidemiology and clinical practice with the EU safety specification, including disease prevalence, demographic distribution, indications, comorbidities, and prescribing patterns. Consider how healthcare system factors (e.g. access, co medications, vaccination programs, Aboriginal and Torres Strait Islander health considerations) could influence the frequency, severity or nature of safety concerns, and document any differences and their implications explicitly in the ASA.
Q5 – What happens if the TGA disagrees with my safety concern classification?
If the TGA does not agree with your proposed safety concern classification, evaluators may request additions, deletions or reclassification of safety concerns during the section 31 information request and evaluation process. Your responses and any agreed changes to the RMP and ASA can become part of the product’s registration conditions, and iterative discussion during evaluation is expected so that the final risk management is appropriate for the Australian context.
Q6 – How often must I update my Risk Management Plan after approval?
There is no universal fixed schedule for RMP updates. You should update the RMP when new safety information emerges, when safety concerns or risk minimisation measures change, when the EU or global RMP is significantly revised, or when the TGA specifically requests an updated RMP or ASA. Proactive maintenance and timely submission of updates demonstrate sound pharmacovigilance practice and compliance with RMP commitments.
Q7 – What records should I maintain to demonstrate RMP implementation?
You should maintain clear records of how RMP commitments are implemented, including: controlled versions and distribution records for educational and risk minimisation materials, training materials and completion logs, signal detection and evaluation documentation, protocols and reports for PASS or effectiveness studies, and any submissions or correspondence with the TGA relating to RMP or ASA updates. These records are key evidence of compliance and are routinely reviewed during pharmacovigilance or GMP/GCP inspections.
References:
- Therapeutic Goods Administration (TGA) – Submitting risk management plans for medicines and biologicals
- Therapeutic Goods Administration (TGA) – Risk management plans for medicines and biologicals
- Therapeutic Goods Administration (TGA) – Template for the Australian-Specific Annex to the Risk Management Plan
- European Medicines Agency (EMA) – Guideline on good pharmacovigilance practices (GVP) Module V – Risk management systems (Rev 2)
- National Centre for Biotechnology Information (NCBI) – Systematic Evaluation of Australian Risk Management Plans (Peer‑reviewed article on RMP content and quality in the Australian regulatory context, 2025)
Disclaimer
This article is provided for educational and informational purposes only. It is intended to support general understanding of regulatory concepts and good practice and does not constitute legal, regulatory, or professional advice.
Regulatory requirements, inspection expectations, and system obligations may vary based on jurisdiction, study design, technology, and organisational context. As such, the information presented here should not be relied upon as a substitute for project-specific assessment, validation, or regulatory decision-making.
We have no commercial relationship with some of the entities, vendors, or software referenced. Any examples are illustrative only, and usage may vary by organisation and their needs.
For guidance tailored to your organisation, systems, or clinical programme, we recommend speaking directly with us or engaging another suitably qualified subject matter expert (SME) to assess your specific needs and risk profile.
Pharmacovigilance Services
At GxpVigilance, we help sponsors and MAHs run TGA-ready pharmacovigilance across Australia—built for clarity, control, and inspection readiness.
- PV system setup & governance (SOPs, SDEAs, oversight)
- AE intake/triage, case processing & regulatory reporting support
- Literature monitoring, signal oversight & safety documentation
- Local PV Contact Person / QPPV / A-PVCP support




