ICH GCP E6(R3) vs ICH GCP E6(R2)
What Actually Changed from ICH GCP E6(R2) to E6(R3)
| Focus Area | Traditional Approach | Modern Approach |
|---|---|---|
Quality Assurance | Retrospective verification | Prospective Quality by Design (QbD) |
Monitoring | Activity-based schedules | Risk-proportionate oversight |
Technology | Implied electronic tolerance | Explicitly technology-neutral |
Accountability | Distributed across sections | Explicit sponsor oversight of Service Providers |
Documentation | Paper-centric references | Data Governance & metadata-aware |
The Structural Shift: Principles and Annexes
- The Principles document contains 11 consolidated GCP principles in outcome-focused language—describing what sponsors must achieve, not precisely how.
- Annex 1 provides detailed guidance for traditional interventional trials. As a result, most current sponsors will anchor their processes here.
- Annex 2, anticipated early 2026, addresses non-traditional designs: pragmatic trials, decentralised trials, and real-world data integration.
- How each principle is addressed in your QMS.
- Where you apply proportionate approaches and the supporting rationale.
- Evidence that flexibility was risk-justified.
Quality by Design Is Now Mandatory Thinking
- A documented CtQ register that teams maintain throughout the trial.
- Evidence that protocol design considered quality proactively.
- Cross-functional input in CtQ identification.
Risk-Based Quality Management Under E6(R3)
- Risk identification – What could go wrong? Where are the critical data points?
- Risk control – What processes or checks mitigate those risks?
- Risk communication – How do teams communicate risks across stakeholders?
- Risk review – How frequently do teams revisit assessments?
- Risk assessments that teams create at study start but never revisit.
- No documented process for risk escalation.
- KRIs and QTLs that teams define but do not actively monitor.
Technology, Decentralisation, and Data Integrity
- Your system validation approach (risk-proportionate).
- Data flow diagrams for each technology component.
- Vendor oversight and qualification evidence.
- ALCOA+ compliance across electronic systems.
Sponsor Oversight Is Sharpened, Not Shared Away
- Written oversight plans (not just service agreements).
- Vendor performance review records.
- Decision logs showing sponsor engagement on quality issues.
- Clear delegation and responsibility matrices.
Where Do You Start? A Practical Transition Pathway
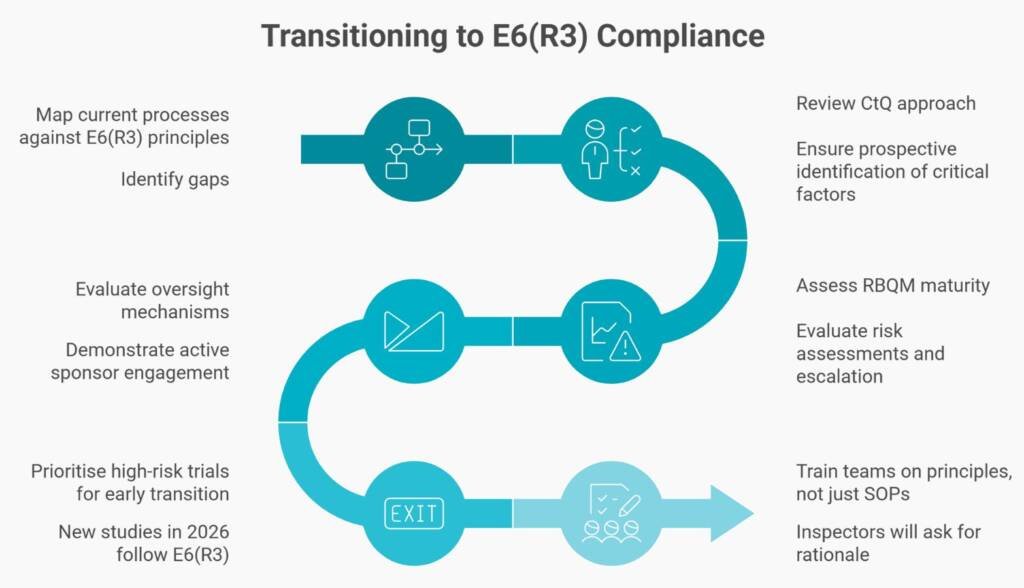
- Step 1: Map your current R2-based processes against the 11 E6(R3) principles. Then identify gaps.
- Step 2: Review your CtQ approach. Do you identify critical factors prospectively with cross-functional input?
- Step 3: Assess your RBQM maturity. Are your risk assessments living documents with defined escalation?
- Step 4: Evaluate your oversight mechanisms. Can you demonstrate active sponsor engagement beyond contract signature?
- Step 5: Prioritise high-risk trials for early transition. New studies starting in 2026 should follow E6(R3) principles from the outset.
- Step 6: Train your teams on principles, not just SOPs. Inspectors will ask staff to explain rationale, not recite procedures.
E6(R3) Readiness Checklist
- You document and maintain a CtQ register for active studies.
- Your team performs risk assessments at study start and reviews them throughout the lifecycle.
- Your RBQM plan aligns with actual trial risks (not a generic template).
- You document oversight plans for CROs and vendors.
- You map data integrity controls (ALCOA+ across electronic systems.
- You document the rationale for the technology validation (proportionate approach).
- You maintain clear delegation and responsibility matrices.
- Your inspection-ready evidence repositories are accessible.
- Staff complete training on E6(R3) principles and rationale.
- Management reviews quality signals and escalation evidence.
Ethics, Participants, and Transparency
What E6(R3) Is Not
- It is not a relaxation of GCP standards.
- It is not permission for unchecked automation without human oversight.
- It is not a reason to reduce documentation or skip validation.
- It is not an excuse to remove human judgment from safety-critical decisions.
Conclusion: Modernisation With Control
Common Questions and Answers
When does ICH E6(R3) become mandatory in Australia?
The TGA expects to adopt ICH E6(R3) in early 2026 with a 12-month transition period. Full enforcement is anticipated from February 2027. During the transition, sponsors may comply with either E6(R2) or E6(R3), but new studies should be designed under R3 principles.
What is the main difference between E6(R2) and E6(R3)?
E6(R3) shifts from checklist compliance to principle-driven quality management. It emphasises prospective quality by design, risk-proportionate oversight, and explicit sponsor accountability—rather than prescriptive procedures applied uniformly regardless of risk.
What are Critical-to-Quality (CtQ) factors?
CtQ factors are elements essential to trial success, participant safety, and data credibility. They are identified during protocol development and used to focus quality efforts on what matters most. Examples include timely safety reporting, accurate endpoint measurements, or protocol adherence at high-risk sites.
Does E6(R3) reduce monitoring requirements?
E6(R3) enables risk-proportionate monitoring, not reduced monitoring. Low-risk elements may receive less intensive oversight, but high-risk elements require more focused attention. Centralised statistical monitoring often replaces routine 100% source data verification.
How does E6(R3) address decentralised clinical trials?
E6(R3) is technology-neutral, explicitly accommodating wearables, eSource, remote visits, and electronic consent. Annex 2 (expected late 2025) will provide detailed DCT guidance. Data integrity expectations increase with technology complexity—audit trails, validation, and oversight must be documented.
What sponsor oversight evidence will inspectors expect?
Inspectors will look for written oversight plans, vendor performance reviews, decision logs showing sponsor engagement, and clear responsibility matrices. The contract alone is insufficient—active, documented oversight throughout the trial is required.
How should we prepare staff for E6(R3) inspections?
Train teams on the 11 E6(R3) principles and the rationale behind your processes. Staff should be able to explain why procedures exist, how risks are managed, and what evidence supports compliance—not just recite SOP steps. Inspectors assess understanding, not memorisation.
Disclaimer
This article is provided for educational and informational purposes only. It is intended to support general understanding of regulatory concepts and good practice and does not constitute legal, regulatory, or professional advice.
Regulatory requirements, inspection expectations, and system obligations may vary based on jurisdiction, study design, technology, and organisational context. As such, the information presented here should not be relied upon as a substitute for project-specific assessment, validation, or regulatory decision-making.
For guidance tailored to your organisation, systems, or clinical programme, we recommend speaking directly with us or engaging another suitably qualified subject matter expert (SME) to assess your specific needs and risk profile.




