In pharmaceutical operations, technical writing is the documented proof of regulatory compliance and patient safety. Inspectors and auditors turn to documentation first to assess whether appropriate controls are in place.
A single transcription error, recording “25.0 g instead of 2.50 g,” creates a patient safety risk. An ambiguous manufacturing instruction, such as “mix thoroughly,” rather than “mix at 200 rpm for 15 minutes at 25°C ± 2°C,” produces batch-to-batch variation that quality cannot defend against. Incomplete deviation reports demonstrate inadequate quality systems when inspectors review them.
Pharmaceutical technical writing is not just administrative. Every SOP, batch production record, clinical study report, and deviation investigation form provides evidence that regulators rely on to confirm patient safety controls and data integrity.
This guide examines what separates adequate documentation from inspection-ready evidence, using six essential characteristics and the ALCOA+ framework that form the data integrity foundation regulators expect.
What Is Pharmaceutical Technical Writing?
Pharmaceutical technical writing encompasses creating, managing, and maintaining documentation across the drug development lifecycle—from research protocols through manufacturing procedures to post-market surveillance. Each document is an official record required for compliance with Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Documentation Practice (GDP).
The scope spans distinct categories. Regulatory documents include CSRs prepared in accordance with the International Council for Harmonisation (ICH) E3 guidelines, Investigator Brochures, study protocols, and Common Technical Document (CTD) modules. Manufacturing documentation comprises SOPs, batch production records, validation protocols, and deviation reports, all aligned with the Pharmaceutical Inspection Co-operation Scheme (PIC/S) guidelines. Clinical documentation includes informed consent forms, case report forms, and safety reports compliant with ICH E6(R3).
| Document Category | Examples | Primary Audience | Regulatory Framework |
|---|---|---|---|
Regulatory Submissions | CSRs, CTD modules, IBs | Health authorities | ICH E3, ICH M2 |
Manufacturing | SOPs, batch records, deviations | Production staff, QA | GMP, PIC/S |
Clinical | Protocols, ICFs, CRFs | Sites, ethics committees | GCP, ICH E6(R3) |
Quality Systems | CAPAs, change controls | Quality teams | ISO 9001, GMP |
Why This Matters
Regulators treat every document as evidence. The distinction between “adequate” and “inspection-ready”
Documentation can determine whether an organisation passes regulatory scrutiny.
The Six Essential Characteristics That Define Excellence
Precision and Accuracy: The Non-Negotiable Foundation
Precision is documenting every fact, measurement, and instruction so nothing is left to interpretation. Accuracy is ensuring every statement is verified as true. They together prevent patient harm and regulatory issues.
Pharmaceutical operations require strict specificity. Compare “Administer in the evening” with “Administer at 8:00 PM ± 30 minutes with 200 mL water at room temperature.” The second eliminates interpretation and ensures consistency across operators, shifts, and sites.
Common documentation errors include transcription mistakes (“105 entered instead of 10.5”), decimal point errors (“25.0 g recorded instead of 2.50 g”), and unit inconsistencies (“mg written when g was measured”). Each represents a potential batch failure or patient safety risk.
What Inspectors Look For: Verified facts with documented sources, consistent measurement units throughout documents, unambiguous procedural steps using action verbs, complete parameter specifications, and absence of vague terms like “approximately” in critical specifications.
Clarity and Concise: Making Complexity Accessible
Clarity means presenting information so your intended audience understands and can execute correctly. Concise means using the fewest words necessary without sacrificing completeness.
The same clinical trial data requires different presentations: a technical CSR for regulatory reviewers, employing specialised terminology; a practitioner-oriented summary for healthcare professionals; and a plain-language synopsis for study participants, avoiding jargon entirely.
For manufacturing SOPs read by operators executing procedures under time pressure, clarity demands step-by-step instructions using imperative language: “Transfer the solution to Tank A” rather than “The solution should be transferred.” Concise matters—excessive wordiness slows execution and increases error risk during production runs.
Regulatory Compliance: The Framework You Cannot Ignore
Pharmaceutical documentation must adhere to frameworks established by the TGA (Australia), FDA (United States), EMA (European Union), and ICH (global harmonisation). Non-compliance triggers warning letters, product recalls, and approval delays.
For electronic records, 21 CFR Part 11 of the FDA establishes requirements to confirm data trustworthiness. For clinical study reports, ICH E3 specifies required sections. For manufacturing documentation, 21 CFR Part 211 and EU GMP mandate comprehensive SOPs covering all GMP-related activities.
Technical writers must maintain current knowledge as regulations evolve—the transition from ICH E6(R2) to E6(R3), the implementation of PIC/S Annex 11, and the adoption of EU Annex 1 (2022) all require documentation updates reflecting new requirements.
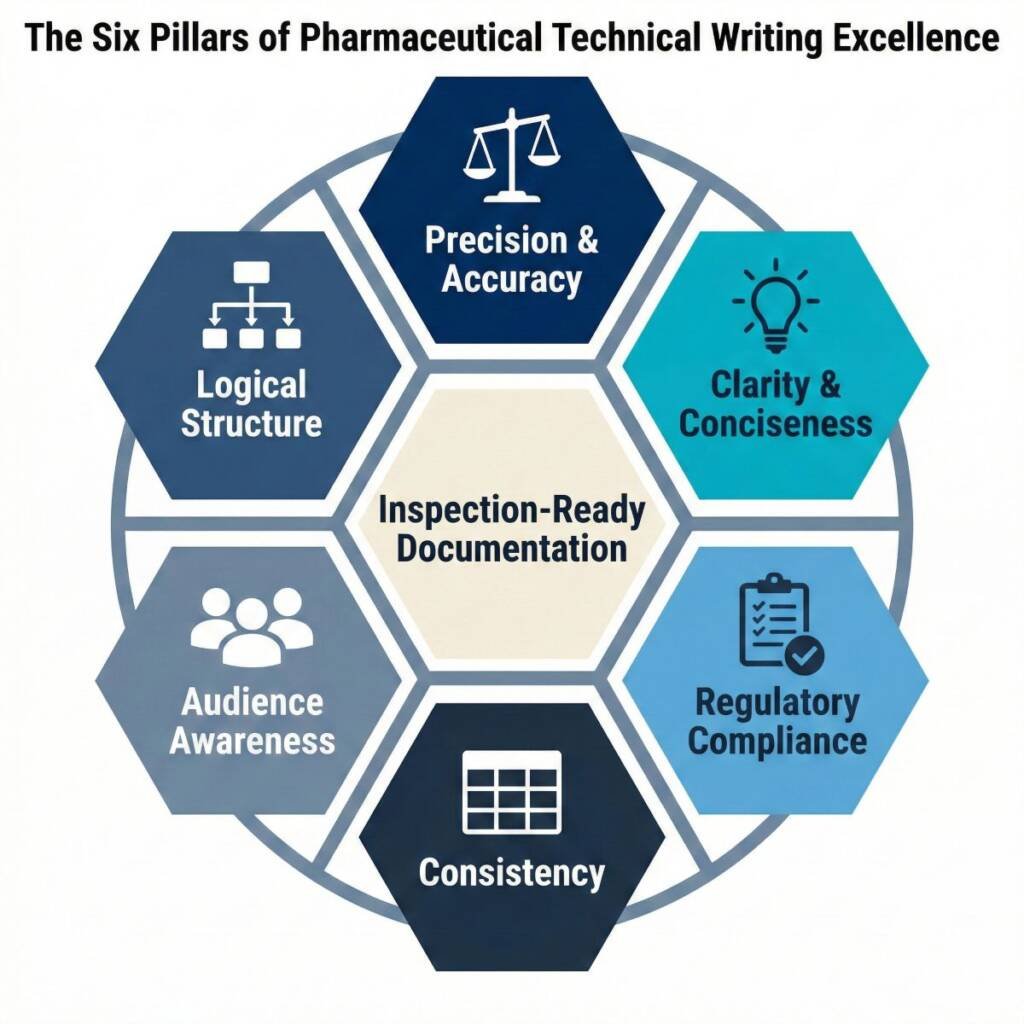
Consistency: Building Trust Through Standardisation
Consistency means using uniform terminology, formatting, and structure across documentation so readers can focus on the content rather than deciphering variations.
Once documents establish terminology—whether “subject” or “participant,” “drug product” or “investigational product”—that terminology must remain consistent. Switching between synonyms can create confusion and lead regulators to believe that different terms represent distinct concepts when they don’t.
Style guides and templates provide essential tools for standardisation, specifying document structure, section headings, font specifications, and formatting standards.
Audience Awareness: Calibrating Technical Depth
Effective pharmaceutical technical writing adjusts complexity, terminology depth, and explanation level based on who will read and use the document.
When writing for regulatory authorities and scientific reviewers, you can employ specialised terminology confidently because readers possess the requisite background. When preparing patient education materials, you must define medical terms, avoid jargon, and use analogies to clarify complex concepts.
Manufacturing SOPs illustrate this clearly. Operators executing procedures need action-oriented steps (“Press the green START button”) rather than theoretical explanations. Quality reviewers reading the same SOPs need rationale sections that explain why the parameters matter.
Logical Structure and Organisation: Creating Clear Information Flow
Well-organised documentation guides readers efficiently through information using clear hierarchies and logical sequences.
Pharmaceutical documents follow structures mandated by regulation. ICH E3 specifies the structure of the clinical study report, including defined sections. SOPs follow standard formats: Title, Purpose, Scope, Responsibilities, Procedure, References, Revision History. This standardisation means reviewers know exactly where to find specific information.
Understanding ALCOA+: The Data Integrity Standard Regulators Expect
Short Answer: ALCOA+ represents 9 principles that form the data integrity foundation: Attributable, Legible, Contemporaneous, Original, Accurate (original ALCOA), plus Complete, Consistent, Enduring, and Available. Regulators expect documented evidence demonstrating compliance with all nine.
The Original ALCOA Principles
- Attributable: Every data entry identifies who performed the action and when. Electronic systems require unique user credentials. Paper records require legible signatures with dates.
- Legible: Records must remain legible throughout the retention period. Use permanent ink for paper records.
- Contemporaneous: Capture data at the time of observation—not reconstructed later. Electronic systems timestamp entries automatically.
- Original: Maintain the original record or a certified true copy, preserving all content and metadata.
- Accurate: Confirm data reflects actual observations without transcription errors.
The “Plus” Extensions
- Complete: Include all data generated—not just results meeting specifications. Document deviations, re-tests, and out-of-specification results with justification.
- Consistent: Maintain data in chronological order, with timestamps that demonstrate logical progression.
- Enduring: Records remain readable throughout required retention periods.
- Available: Confirm records remain accessible for review. Regulators expect to see data within minutes of requesting it during inspections.

What Skills Do Pharmaceutical Technical Writers Need?
Short Answer: Pharmaceutical technical writers require competencies across writing proficiency, scientific knowledge, regulatory expertise, and interpersonal capabilities.
Essential Skills:
- Writing Proficiency: Exceptional grammar and syntax, medical terminology knowledge, plain language translation skills, and active versus passive voice mastery
- Scientific Knowledge: Understand life sciences, drug development, clinical trials, and therapeutic areas.
- Regulatory Expertise: Know TGA, FDA, EMA, ICH, 21 CFR Part 11, and GMP/GCP/GDP/GVP principles, plus regional variations.
- Interpersonal Skills: Focus on detail, manage time, collaborate across functions, and adapt to regulatory changes.
Common Challenges and Practical Solutions
- Translating Complex Scientific Information: Scientists communicate in specialised language. Your job is to translate this into documents that regulators, healthcare professionals, and patients can understand without losing scientific accuracy.
- Solution: Consult subject matter experts early in the document development process. Develop a glossary of technical terms. Use visual aids such as flowcharts, diagrams, and tables to efficiently convey complex processes.
- Maintaining Currency with Evolving Regulations: Regulatory frameworks are continually evolving. Staying current demands deliberate effort.
- Solution: Subscribe to regulatory intelligence services tracking guidance updates. Join professional associations offering education programmes. Establish internal processes where regulatory affairs teams communicate relevant changes. Schedule quarterly reviews of key guidance documents.
- Managing Version Control: Without rigorous version control, you risk working on outdated versions or being unable to demonstrate document history during inspections.
- Solution: Implement electronic document management systems providing automated workflows, version control, and comprehensive audit trails. Establish version numbering conventions—whole numbers (1.0 → 2.0) for substantive revisions, decimal changes (1.0 → 1.1) for drafts.
Your Path to Documentation Excellence
Technical writing in pharmaceutical operations is a critical control point. Documentation demonstrates patient safety controls, regulatory compliance, and operational consistency—the evidence regulators review during inspections.
As documentation complexity grows in the pharmaceutical industry, novel therapeutic modalities demand new approaches, while global regulatory harmonisation requires consistency across jurisdictions.
Amid these challenges, digital transformation introduces the need for validated electronic documentation controls. As a result, technical writing capability becomes essential for regulatory success.
Take decisive action: Assess your organisation’s documentation practices against the six characteristics: precision, clarity, regulatory compliance, consistency, audience awareness, and logical structure. Pinpoint the one area where targeted improvement will most powerfully enhance inspection readiness. Commit to making that change now.
Document your current state. Define your desired future state. Start implementing targeted changes, and systematically record clear evidence of each improvement.
Common Questions and Answers
What distinguishes pharmaceutical technical writing from general technical writing?
Pharmaceutical technical writing operates within strict regulatory frameworks where documentation serves as legal evidence of GxP compliance; documentation errors directly impact patient safety, trigger recalls, regulatory sanctions, and legal liability in ways that general technical writing does not.
How does the ALCOA++ framework apply to everyday documentation?
ALCOA++ requires all pharmaceutical records be Attributable (identifying who), Legible (permanently readable), Contemporaneous (real-time entry), Original (authentic), Accurate (true values), Complete (all necessary data), Consistent (standardized), Enduring (retained), Available (retrievable), and Traceable (with documented audit trails), ensuring data integrity across paper and electronic systems.
What qualifications do pharmaceutical technical writers typically need?
Pharmaceutical technical writers typically hold bachelor’s or advanced degrees in life sciences, pharmacy, or chemistry; require exceptional writing ability, understanding of drug development, and regulatory knowledge; and benefit significantly from professional certifications such as Medical Writer Certified (MWC®), RAC, or BELS.
How should technical writers handle corrections in pharmaceutical documents?
Paper records require single-line strikethroughs preserving original text visibility, followed by corrected entry, initials, date, and documented reason; electronic systems must maintain tamper-evident audit trails automatically capturing who changed what, when, and why per 21 CFR Part 11, with no obliteration of original entries ever permitted.
What makes an SOP effective from a regulatory perspective?
Effective SOPs employ clear titles, standardized structure, imperative language, robust version control with documented approval, evidence of comprehensive staff training on current versions with competency verification, and most critically, demonstrated alignment between written procedures and actual operational practices verified through regular reviews and compliance records.
How do I maintain regulatory knowledge as frameworks evolve?
Subscribe to AI-powered regulatory intelligence platforms monitoring TGA, FDA, EMA, and other authorities; join professional associations (ARCS, AMWA, RAPS) offering continuing education; establish internal knowledge-sharing processes where regulatory changes are communicated and translated into operational impact; and schedule regular reviews of ICH guidance and agency-specific documents.
What role does audience awareness play in regulatory submissions?
Regulatory reviewers represent diverse specializations (chemistry, nonclinical, clinical pharmacology, medical, biometrics, safety)—each assessing specific modules with distinct criteria—requiring submissions to calibrate technical depth appropriately for each reviewer type while maintaining absolute consistency in terminology, data, and conclusions across all modules to prevent regulatory questions and delays.
References
- International Council for Harmonisation (ICH) – ICH E6(R3) Guideline for Good Clinical Practice (Final Version, Adopted 6 January 2025)
- International Council for Harmonisation (ICH) – ICH E3 Structure and Content of Clinical Study Reports (Step 5, Adopted 30 June 1996)
- U.S. Food and Drug Administration (FDA) – 21 CFR Part 11 – Electronic Records; Electronic Signatures – Scope and Application (Guidance for Industry, U.S. Department of Health and Human Services, 2003)
- Pharmaceutical Inspection Co-operation Scheme (PIC/S) – Guide to Good Manufacturing Practice for Medicinal Products (PE 009-16, Latest Edition)
- International Council for Harmonisation (ICH) – ICH Q9(R1) Guideline: Quality Risk Management (Step 4, Adopted 19 December 2022)
Disclaimer
This article is provided for educational and informational purposes only. It is intended to support general understanding of regulatory concepts and good practice and does not constitute legal, regulatory, or professional advice.
Some images used in this article have been generated or enhanced using AI for illustrative and educational purposes and do not represent real client records, systems, or regulated source data.
Regulatory requirements, inspection expectations, and system obligations may vary based on jurisdiction, study design, technology, and organisational context. As such, the information presented here should not be relied upon as a substitute for project-specific assessment, validation, or regulatory decision-making.
For guidance tailored to your organisation, systems, or clinical programme, we recommend speaking directly with us or engaging another suitably qualified subject matter expert (SME) to assess your specific needs and risk profile.




