AI in Antimicrobial Stewardship: Safe, Fast, Human-Led, and Evidence-Grounded
The future of AI in antimicrobial stewardship is rapidly taking shape. Health systems are moving toward real-time resistance surveillance, evidence-grounded clinical decision support, and automated workflows that ease administrative burden. Yet one principle remains: antimicrobial stewardship will always depend on expert human judgment. AI will enhance situational awareness and accelerate evidence retrieval, but clinicians such as pharmacists, microbiologists, and infectious disease specialists will continue to make the final decisions that impact patient safety.
This article merges emerging practice, future trends, and governance expectations into a comprehensive view of how AI will transform antimicrobial stewardship by 2030, without compromising clinical reasoning, transparency, or ethical responsibility.
The Case for AI in Stewardship Today
Antimicrobial resistance is advancing faster than current systems can monitor. Traditional surveillance methods are largely retrospective and labour-intensive, and guidelines can lag behind local epidemiology. AI does not replace stewardship expertise, but enables teams to detect resistance patterns earlier, interpret data faster, and access validated information instantly.
For overstretched stewardship teams, AI offers practical support:
- Faster access to guidelines, antibiograms, and product information
- Earlier detection of emerging resistance trends
- Improved triage of high-risk patients
- Automated literature screening and evidence retrieval
- Reduction in repetitive administrative tasks
- Greater consistency in documentation and reasoning trails
The intention is not automation for its own sake. It is to give clinicians more time for complex thinking, nuanced interpretation, and patient-centred decision-making.
What Is AI in Antimicrobial Stewardship?
AI in antimicrobial stewardship refers to systems that surface evidence, synthesise data, highlight anomalies, and guide decision-making while preserving human oversight. Unlike consumer chatbots, stewardship AI must be transparent, audit-ready, and based on validated sources.
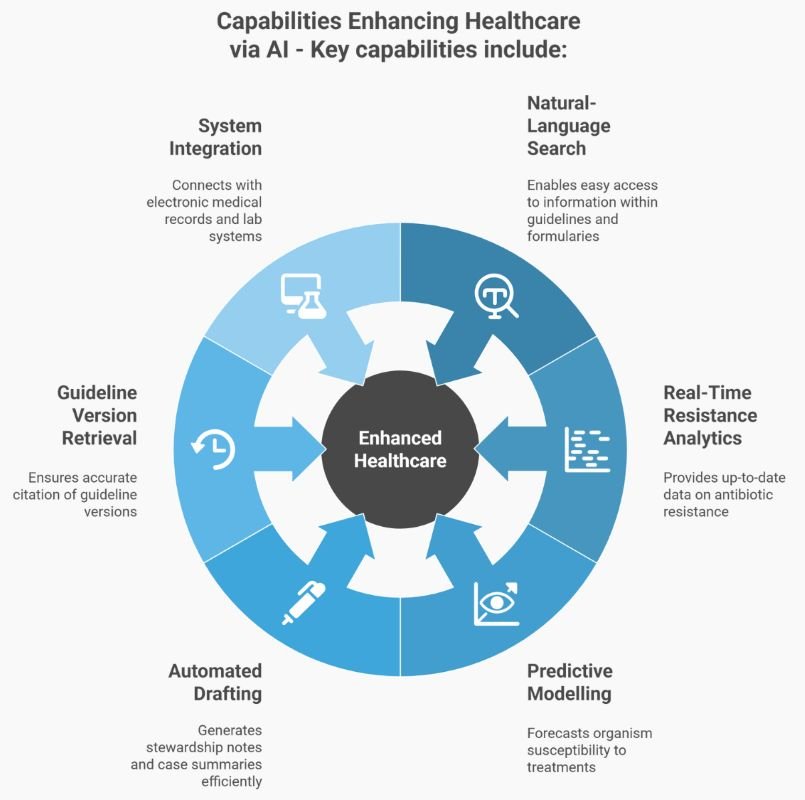
AI accelerates intelligence gathering. Humans use clinical judgment to turn intelligence into safe care.
How AI-Enabled Surveillance Works
AI-driven surveillance shifts stewardship from retrospective reporting to real-time foresight. These systems integrate data from electronic health records, microbiology laboratories, genomic sequencing platforms, and environmental surveillance such as wastewater testing.
Modern systems can:
- Detect unusual susceptibility patterns within hours
- Identify clusters or outbreaks days before traditional methods
- Predict resistance risk for individual patients
- Analyse years of local epidemiology to refine empirical therapy
- Combine One Health datasets across human, animal, agriculture, and environmental domains
As demonstrated by initiatives such as OUTBREAK in Australia, integrated surveillance platforms reveal patterns not visible to the unaided eye. Machine learning models trained on longitudinal patient data can match or exceed clinician performance in selecting appropriate empirical therapy, often choosing narrower-spectrum agents.
However, no matter how advanced surveillance technology becomes, the interpretation of these signals still relies on human reasoning. AI highlights patterns. Clinicians contextualise them.
Why Human Judgment Remains Central
Even with rapid advances, AI cannot weigh the complex variables that shape antimicrobial decisions. Factors such as organ function, pregnancy, allergies, immune suppression, comorbidities, pharmacokinetics, or long-term ecological consequences remain beyond the capacity of autonomous systems.
Effective stewardship requires:
- Pharmacists to optimise dosing, check interactions, and oversee daily therapy
- Microbiologists to interpret culture accuracy and organism significance
- Infectious disease physicians need to balance severity, risk, and treatment duration
- Clinical nursing teams to detect deterioration, toxicity, or adverse response
AI supports these professionals by reducing noise, accelerating information gathering, and ensuring all evidence is current. Clinical responsibility never shifts to an algorithm.
The Role of RAG Architectures
Stewardship relies on transparent, traceable evidence. This makes Retrieval-Augmented Generation (RAG) essential. Unlike generic large language models that may hallucinate information, RAG systems ground every output in validated sources.
In practice, this means a clinician can ask:
“What is the recommended empirical therapy for hospital-acquired pneumonia in a patient with recent carbapenem exposure and renal impairment?”
Within seconds, a RAG system retrieves:
- Local antibiogram data
- Institutional treatment guidelines
- TGA-approved product information
- Allergy records
- International consensus statements (e.g., IDSA, ESCMID)
Transparency is mandatory. Stewardship teams must be able to verify the source and relevance of every AI-generated recommendation.
Building Trustworthy AI Ecosystems in Stewardship
Adoption depends not only on accuracy but on clinician trust. A trustworthy AI ecosystem encompasses governance, collaboration, validation, and human oversight that work in tandem.
Data Provenance and Transparency
Clinicians must know exactly which documents—such as guidelines, product information, and antibiograms—feed the AI. Version control, automated updates, and citation accuracy are essential to establishing confidence.
Continuous Model Monitoring
Resistance patterns evolve quickly. Stewardship AI requires:
- Drift detection
- Regular validation
- Scheduled retraining
- Performance dashboards
- Bias assessment
These safeguards ensure outputs remain clinically valid over time.
Interoperability
AI must integrate seamlessly into existing workflows, including:
- EHRs and ePrescribing systems
- LIMS and microbiology platforms
- Clinical decision-support tools
- Stewardship dashboards
The more context-aware the system, the more clinicians trust its recommendations.
Shared Ownership
AI succeeds when stewardship teams shape the tool’s behaviour. Involving pharmacists, microbiologists, and ID physicians in design and piloting fosters ownership and transforms AI from an external tool into an embedded clinical partner.
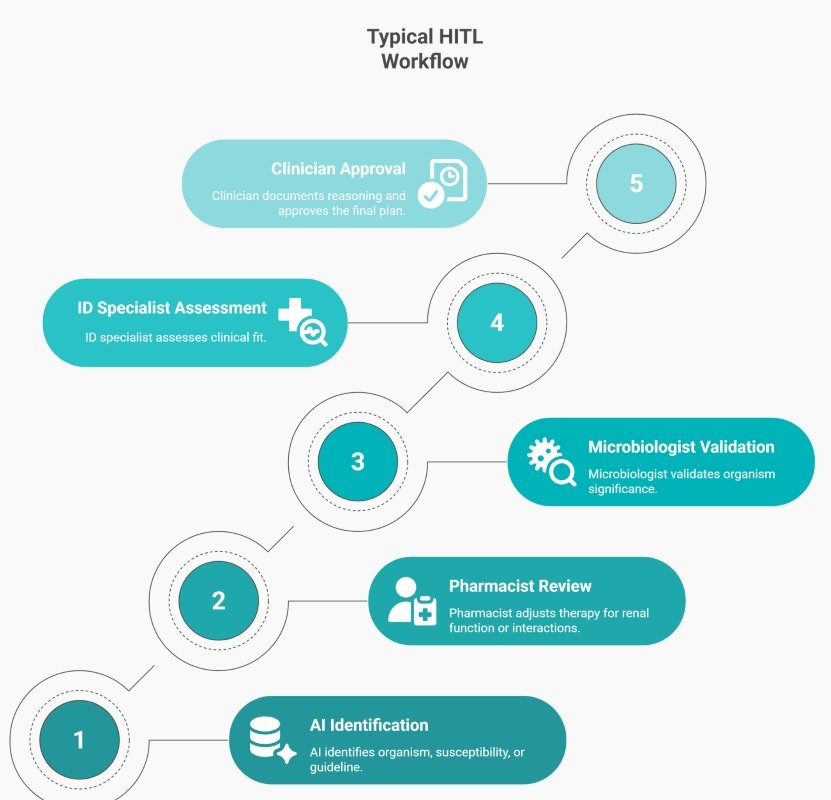
Human-in-the-Loop Decision Support
Human-in-the-loop (HITL) governance ensures that AI recommendations never influence patient care without clinical verification. This is especially critical in antimicrobial stewardship, where small errors can carry significant consequences.
This model preserves accountability while reducing review time. AI accelerates; humans decide.
What Stewardship Will Look Like in 2030
By 2030, AI will be quietly embedded into everyday workflows. Clinicians will not “use AI”; they will simply work in systems where AI support is available when needed.
Expect:
- Real-time surveillance dashboards
- Automated evidence retrieval
- Transparent, citation-backed recommendations
- Faster case prioritisation
- Predictive modelling for patient-specific risks
- Interoperable stewardship platforms
- Clear documentation trails
- Reduced variation in practice
The essence of stewardship remains unchanged. AI amplifies expertise; it does not replace it.
Conclusion & Next Steps
AI in antimicrobial stewardship strengthens clinical practice by offering speed, clarity, and early detection of risk. It reduces administrative burden, supports evidence-based care, and enhances coordination across multidisciplinary teams. Stewardship must remain clinician-led, ethically governed, and human-centred. The future is not AI-driven; it is AI-enabled.
To adopt AI safely, teams need governance, transparent data sources, validation protocols, and human-in-the-loop review. These foundations ensure stewardship programs remain rigorous, accountable, and ready for the future.
References
- How AI can help us beat AMR
- Retrieval-augmented generation for generative artificial intelligence in healthcare
- AI-Based Clinical Decision Support System for Antibiotic Prescribing
- ISO/IEC 42001 – AI Management System
- Artificial Intelligence & Antimicrobial Stewardship: Insights from Experts
- TGA – AI and medical device software regulations