The pharmacovigilance consultant in Australia occupies a distinctive position within the pharmaceutical ecosystem. These professionals serve as the operational bridge between regulatory obligation and practical compliance, ensuring medicines remain safe throughout their lifecycle while sponsors meet TGA requirements. As AI enters pharmacovigilance workflows, the role is changing in ways that matter for sponsors, practitioners, and patient safety alike.
Understanding who these consultants are, what they actually do, and how the profession is adapting to an increasingly automated environment matters whether you are a sponsor engaging external expertise, a professional considering this career path, or a practitioner navigating the AI transition. This article provides practical clarity on each dimension.
Who Becomes a Pharmacovigilance Consultant in Australia?
The typical pharmacovigilance consultant in Australia holds a degree in pharmacy, biomedical science, or nursing, often with postgraduate qualifications in clinical research or regulatory affairs. Registration as a pharmacist, doctor or nurse with AHPRA is common but not mandatory. What distinguishes senior consultants—particularly those acting as Qualified Persons for Pharmacovigilance (QPPV)—is deep experience: typically 8-10 years spanning case processing, signal detection, aggregate reporting, and direct interaction with the TGA.
Career entry usually follows one of three paths. Clinical practitioners transition from hospital or community pharmacy seeking analytical work with predictable hours and remote work flexibility. Clinical research professionals pivot from site-monitoring or data-management roles, recognising PV’s favourable work-life balance compared to site-based clinical operations. Life sciences graduates enter the field of pharmacovigilance through entry-level drug safety positions at pharmaceutical companies or CROs, drawn by competitive salaries and meaningful public health impact.
What drives them? Three factors appear consistently across the profession.
- First, patient safety operates as a professional north star. Consultants describe deep satisfaction in detecting signals that prevent population-level harm—the work operates at a systemic level, identifying safety concerns that protect millions rather than treating individual patients.
- Second, the intellectual complexity attracts analytical minds: causality assessment requires synthesising sparse clinical data, pharmacology knowledge, and regulatory judgement. Each case presents unique clinical puzzles requiring medical reasoning and contextual analysis.
- Third, flexibility matters significantly. Independent consultants can work remotely, manage multiple clients, and control their schedules in ways traditional pharmacy roles rarely permit—a considerable draw for mid-career professionals or parents seeking lifestyle compatibility.
The most effective consultants combine technical precision with communication ability. They must translate complex safety data for clinical teams, commercial stakeholders, and regulators—often simultaneously. A consultant might explain MedDRA coding nuances to a data team in the morning and present benefit-risk assessments to executive leadership in the afternoon. Ethical fortitude matters too: QPPVs face implicit pressure from commercial teams to minimise the significance of adverse events, and the role requires the willingness to push back on clients when patient safety demands it.
What Does a Pharmacovigilance Consultant in Australia Do?
The pharmacovigilance consultant in Australia handles the “what” of drug safety monitoring across the entire medicine lifecycle. Their work is post-marketing-focused, complementing clinical trial data with real-world surveillance that continues as long as a product remains on the market.
Regulatory framework context: Australian sponsors must nominate an Australian Pharmacovigilance Contact Person (A-PVCP) within 15 days of their first ARTG listing. This is a legal requirement under the Therapeutic Goods Act 1989. However, the TGA’s Pharmacovigilance Inspection Program assesses not just paperwork but functional capability—system architecture, decision-making authority, and demonstrable oversight. This gap between the minimal statutory “contact” and the rigorous functional “expert” creates the market for consultants.
International sponsors face an additional challenge: the A-PVCP must reside in Australia. A company cannot fulfil this requirement with a global head of safety based in the US or Europe. This residency mandate creates permanent structural demand for Australian-based consultants that cannot be offshored to lower-cost jurisdictions.
Core responsibilities include:
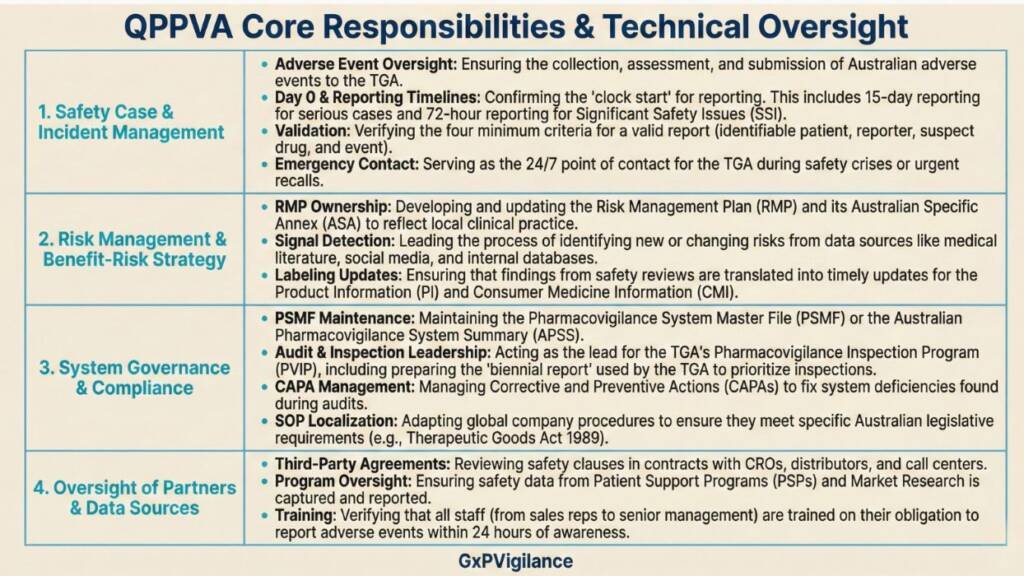
The “Fractional QPPV” Model: Many Australian biotechs and international sponsors use consultants as fractional QPPVs. A single consultant manages 3-10 clients, providing named oversight at a fraction of the cost of a full-time hire. This model reduces compliance costs by approximately 80% compared to traditional internal staffing while providing access to senior expertise. A pre-revenue biotech with one Phase II product faces the same regulatory obligations as a top-tier multinational; the fractional model makes this manageable.
How the Pharmacovigilance Consultant in Australia Is Adapting to AI
AI is not replacing consultants in Australia. It is shifting their value proposition from data processing to oversight and validation—a significant distinction that sponsors and practitioners need to understand clearly.
Example: Historically, 60-80% of a consultant’s billable time was spent on manual tasks: reading emails, entering data into safety databases, selecting MedDRA codes, and drafting case narratives. AI-enabled workflows now automate much of this work. Natural language processing extracts adverse event elements from unstructured sources—such as physician notes, call centre transcripts, and patient emails. Machine learning drafts case narratives and suggests coding. Literature surveillance tools triage thousands of abstracts in minutes, presenting only high-probability articles for human review.

What AI handles well:
- Automated case triage (serious/non-serious, expected/unexpected classification)
- Duplicate detection across multiple reporting channels
- Literature screening with relevance scoring
- Initial narrative drafting from structured data fields
- MedDRA coding suggestions based on reporter descriptions
- Causality assessment requires clinical judgement
- Medical significance evaluation in ambiguous cases
- Regulatory strategy decisions and benefit-risk interpretation
- Inspection defence and stakeholder communication
- Validating AI outputs for accuracy and “hallucinations”
This creates the “Human-in-the-Loop” operational model now standard in compliant AI pharmacovigilance. The TGA and other global regulators mandate that a qualified human must retain ultimate responsibility for the safety system. The consultant’s role shifts from writing reports to auditing AI outputs, checking for hallucinations, and ensuring medical logic holds.
Consultants are becoming prompt engineers—encoding their expertise into workflow logic—and validators, proving AI tools are fit for purpose under TGA expectations. Under GAMP 5 principles, AI tools often require validation documentation demonstrating they produce reliable, reproducible outputs. This validation work is itself becoming a new service line.
Practical checklist for sponsors evaluating PV consultants in 2026:
Verify Australian residency (non-negotiable for A-PVCP designation)
- Confirm direct TGA inspection experience.
- Assess therapeutic area expertise relevant to your products.
- Review their approach to AI tool validation (GAMP 5 alignment)
- Understand their oversight model for AI-generated outputs.
- Clarify liability coverage and professional indemnity insurance.
- Check their continuous professional development (ARCS, ISoP membership)
Business Model Implications for Consultants
The shift presents a billing paradox. If AI reduces case processing time by 80%, the traditional hourly billing model collapses. Consultants are adapting by moving toward value-based retainers and subscription models—selling “compliance-as-a-service” rather than charging by the hour for data entry.
New service lines are emerging: AI tool validation, workflow design, and training sponsors’ internal teams to use AI tools compliantly. Consultants who can design AI-integrated safety systems, write validation documentation, and apply critical human judgment to automated outputs command premium positioning.

What This Means for You
If you are a sponsor engaging a pharmacovigilance consultant in Australia, focus on functional capability rather than title alone. The gap between a named contact and genuine QPPV expertise is where inspection findings occur. Ask how they approach AI validation, understand their oversight model for automated outputs, and verify they can demonstrate TGA inspection readiness.
If you are considering PV consulting as a career path, recognise that data entry skills are becoming commoditised. Differentiation lies in clinical judgement, regulatory expertise, AI fluency, and the ability to communicate complex safety information across diverse audiences.
If you are already practising, invest in validating AI tools, building competencies, and developing prompt engineering. The profession is migrating from “processing” to “oversight and design.” The consultants who thrive will be those who can architect AI-integrated systems while maintaining the human judgment that regulators—and patients—require. Patient safety remains the anchor. AI speeds up and standardises pharmacovigilance, but it does not replace the clinical reasoning that transforms data into protection.
Pharmacovigilance Consultant – Common Questions and Answers
What qualifications are required to become a PV consultant in Australia?
Most pharmacovigilance consultants in Australia have at least a bachelor’s degree in pharmacy, medicine, nursing, or life/biomedical sciences, often combined with formal PV or GxP training. While medical qualifications are not legally required for the person overseeing PV, TGA guidance expects access to appropriate clinical and medical expertise for safety assessments. Senior consultants typically bring 8–10+ years’ experience spanning case processing, signal detection, aggregate reporting, and direct interaction with regulators.
What is the difference between an A-PVCP and a QPPV in Australia?
The Australian pharmacovigilance contact person (A-PVCP) is a legal requirement under the Therapeutic Goods Act 1989. Sponsors must nominate a named person in Australia as the primary PV contact and notify the TGA of their details within 15 days of ARTG entry or any change. The Qualified Person for Pharmacovigilance (QPPV or QPPVA) is a functional role describing the expert who oversees the entire Australian PV system and ensures compliance. In practice, the same individual can act as both roles, and TGA inspections assess against QPPV-level expectations.
Will AI replace pharmacovigilance consultants?
No. AI supports automation of routine tasks such as case intake assistance, coding, de-duplication, and literature screening, but sponsors remain accountable for clinical judgement, causality assessment, regulatory strategy, and inspection defence. The consultant role is shifting toward oversight, validation, and AI-enabled process design, increasing the value of experienced professionals.
What does a typical day look like for a PV consultant?
A typical day includes reviewing new adverse event reports, assessing seriousness and reporting timelines, performing signal detection and literature surveillance, collaborating with clinical, regulatory, and medical teams, preparing aggregate reports, and responding to urgent or emerging safety issues.
How do PV consultants stay current with regulatory changes?
Consultants maintain regulatory awareness through professional societies and conferences such as ARCS Australia and ISoP, ongoing education and specialist PV or GxP certifications, and continuous monitoring of regulatory communications from agencies including the TGA, FDA, and EMA. AI governance and validation training is increasingly important.
Why do sponsors choose independent consultants over CROs for PV services?
Sponsors often choose independent consultants because they provide direct access to senior expertise at a lower overall cost than large CROs, frequently via a fractional QPPV/QPPVA model. This approach allows flexible scaling around case volume, inspections, audits, and aggregate reporting cycles.
Who can legally act as the Australian pharmacovigilance contact person (A-PVCP)?
The A-PVCP must be a person in Australia nominated by the sponsor as the primary pharmacovigilance contact, with their details notified to the TGA within 15 calendar days of ARTG entry or any change. The individual should have sufficient PV knowledge and authority to coordinate safety communications with the TGA.
Do I legally need a QPPV in Australia, or only an A-PVCP?
Australian legislation explicitly requires an A-PVCP but does not define a QPPV in law. In practice, the TGA expects a designated individual with QPPV-like oversight of the pharmacovigilance system, and most sponsors appoint a QPPVA to meet inspection expectations.
What core competencies should an aspiring PV consultant develop beyond technical drug-safety skills?
Beyond pharmacology and PV regulations, aspiring consultants should develop strong communication skills, stakeholder management, risk-based thinking, data literacy, and audit and inspection readiness. Experience with safety databases, MedDRA coding, aggregate report authoring, and TGA PV guidance significantly strengthens a consulting profile.
References
- Therapeutic Goods Administration (TGA) – Pharmacovigilance responsibilities of medicine sponsors
- Therapeutic Goods Administration (TGA) – Pharmacovigilance responsibilities of medicine sponsors (PDF) (Australian Government Department of Health and Aged Care, 31 July 2023)
- GxP Vigilance – QPPVA & Pharmacovigilance Roles in Australia (2025 Guide) (Practical explanation of Australian QPPVA function, linkage to A‑PVCP and TGA requirements, 3 November 2025)
- European Medicines Agency (EMA) – Guideline on good pharmacovigilance practices (GVP) Module I – Pharmacovigilance systems and their quality systems
Disclaimer
This article is provided for educational and informational purposes only. It is intended to support general understanding of regulatory concepts and good practice and does not constitute legal, regulatory, or professional advice.
Regulatory requirements, inspection expectations, and system obligations may vary based on jurisdiction, study design, technology, and organisational context. As such, the information presented here should not be relied upon as a substitute for project-specific assessment, validation, or regulatory decision-making.
We have no commercial relationship with some of the entities, vendors, or software referenced. Any examples are illustrative only, and usage may vary by organisation and their needs.
For guidance tailored to your organisation, systems, or clinical programme, we recommend speaking directly with us or engaging another suitably qualified subject matter expert (SME) to assess your specific needs and risk profile.
Pharmacovigilance Services
At GxpVigilance, we help sponsors and MAHs run TGA-ready pharmacovigilance across Australia—built for clarity, control, and inspection readiness.
- PV system setup & governance (SOPs, SDEAs, oversight)
- AE intake/triage, case processing & regulatory reporting support
- Literature monitoring, signal oversight & safety documentation
- Local PV Contact Person / QPPV / A-PVCP support




