Quality management systems in Australian clinical trials represent the structural foundation that protects participants and preserves data integrity. Consequently, sponsors bear direct accountability for trial quality, regardless of how many activities they delegate to contract research organisations (CROs) or vendors.
The Therapeutic Goods Administration (TGA) expects sponsors to demonstrate that they protect participant rights, safety, and well-being while generating reliable trial data. With ICH GCP E6(R3) now adopted by the TGA—effective 13 January 2026—these expectations formalise into explicit principles emphasising quality by design, proportionality, and documented oversight.
This article addresses what these requirements mean in practice for sponsors in Australia. You will understand the core components of a sponsor QMS, common failure points, and how to build systems that withstand inspection scrutiny.
Quality Management Systems in Australian Clinical Trials: The Regulatory Framework
Sponsors conducting clinical trials in Australia operate within overlapping governing frameworks. Each framework reinforces quality management obligations:
ICH GCP E6(R3): Principle 6 requires sponsors to build quality into scientific and operational design. Similarly, Principle 7 expects a proportionate process. Additionally, Principle 10 clarifies that sponsors retain responsibility even when they delegate activities.
NHMRC National Statement: Establishes ethical standards requiring scientifically sound designs that protect participants.
TGA Clinical Trial Notification (CTN) and Clinical Trial Approval (CTA) Schemes: Allow trials to proceed after ethics approval and TGA notification, placing greater self-regulatory responsibility on sponsors.
Many sponsors misunderstand one point: delegation does not transfer responsibility. When a sponsor engages a CRO to manage monitoring or data management, the CRO executes the work, but accountability for quality and integrity remains with the sponsor. Regulators assess systems and evidence, not intentions. An inspector will ask: “How did you maintain oversight?” rather than “Did you trust your CRO?”

What Is a Quality Management System in Clinical Trials?
A QMS in the clinical trial context comprises the organised structure of policies, procedures, responsibilities, and processes that ensure consistent conduct and controlled documentation across the trial lifecycle. Importantly, quality management systems in Australian clinical trials serve purposes extending beyond compliance:
Consistent trial conduct across sites and personnel
Controlled documentation with clear version management
Systematic risk identification and mitigation
Evidence of ongoing improvement
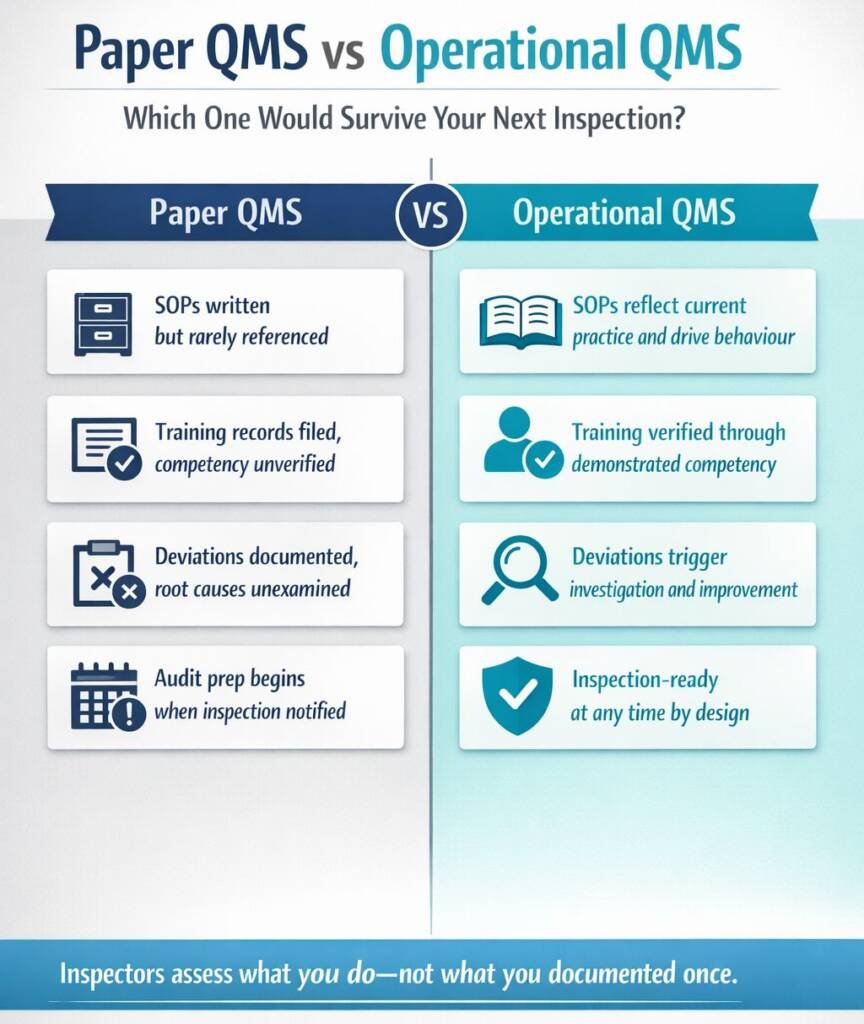
The distinction between a paper QMS and an operational QMS is important. A paper QMS exists in documented form, with SOPs written, training records filed, and processes described, but may not reflect how work actually gets done. An operational QMS is embedded in daily practice: staff follow procedures because they understand them, deviations trigger investigation, and feedback improves the system.
Sponsors should ask: “If an inspector walked through our operations today, might they see our documented system in action—or gaps between what we say and what we do?”
Core Components of a Sponsor Clinical Trial QMS
Sponsors must address five interdependent components to build a functional quality management system:
Governance and Oversight
The sponsor oversight model should be clearly defined, specifying accountability for trial quality, decision escalation processes, and leadership review of CRO performance. Governance structures must be documented and actively implemented, rather than existing solely as theoretical charts.
Document Control and SOP Framework
A controlled document hierarchy should be established, incorporating clear versioning, approval pathways, and distribution processes. Standard operating procedures must reflect current practices and remain accessible to relevant personnel. Controlled documents provide the evidence trail required by inspectors.
Training and Competency Management
Role-based training on Good Clinical Practice and protocol-specific requirements must be documented with evidence of completion. Attendance alone is insufficient; sponsors should also verify competency to ensure personnel understand and can apply the training.
Vendor Qualification and Oversight
CAPA and Deviation Management
- Governance structure documented and communicated.
- SOPs are current, controlled, and accessible.
- Training records complete with competency evidence
- Vendor qualification files are maintained.
- The CAPA system is operational with effectiveness verification.
Types of Quality Systems Used by Sponsors
Sponsors adopt different QMS models depending on organisational size and trial portfolio:
| Model | Typical User | Key Characteristics | Considerations |
|---|---|---|---|
Integrated Corporate QMS | Large pharma, global sponsors | Centralised system covering all trials | Consistent but may require local adaptation |
Trial-Specific QMS | Small–mid sponsors, virtual biotechs | Developed for individual programs | Flexible but risk of inconsistency |
Hybrid Model | Sponsor + CRO shared systems | Combined elements from both parties | Requires clear responsibility mapping |
The choice depends on organisational maturity and trial complexity. Virtual biotechs relying heavily on CROs must remain vigilant: using a CRO’s QMS does not eliminate sponsor obligations. The sponsor must understand, oversee, and defend the quality system as their own.
Consequences of an Absent or Ineffective QMS
When absent or ineffective, quality management systems in Australian clinical trials lead to predictable failures:
- Repeated protocol deviations occur without root cause analysis.
- CRO oversight is inadequate, as shown by unreviewed monitoring reports.
- Documentation and traceability are poor, making it impossible for staff to reconstruct records.
- Ethics non-compliance occurs when teams fail to consistently follow consent processes.
Regulatory consequences may include inspection findings that require corrective action or even trial suspension. More importantly, an absent or ineffective QMS undermines data credibility and raises doubts about whether trial results can support regulatory decisions.
Inspectors follow a clear principle: absence of evidence is evidence of absence. If sponsors cannot provide documentation of oversight, inspectors will assume oversight did not occur.
Quality by Design: Building Quality Into Clinical Trials
ICH E6(R3) Principle 6 formalises a key understanding: quality cannot be added to a trial after it begins. Sponsors must design quality into the protocol, data collection, and monitoring from the start.
Quality by Design (QbD) starts by identifying Critical-to-Quality (CtQ) factors, which are essential for participant protection and data integrity. Focusing on CtQ factors during protocol development helps sponsors avoid unnecessary complexity, deviations, and wasted resources.
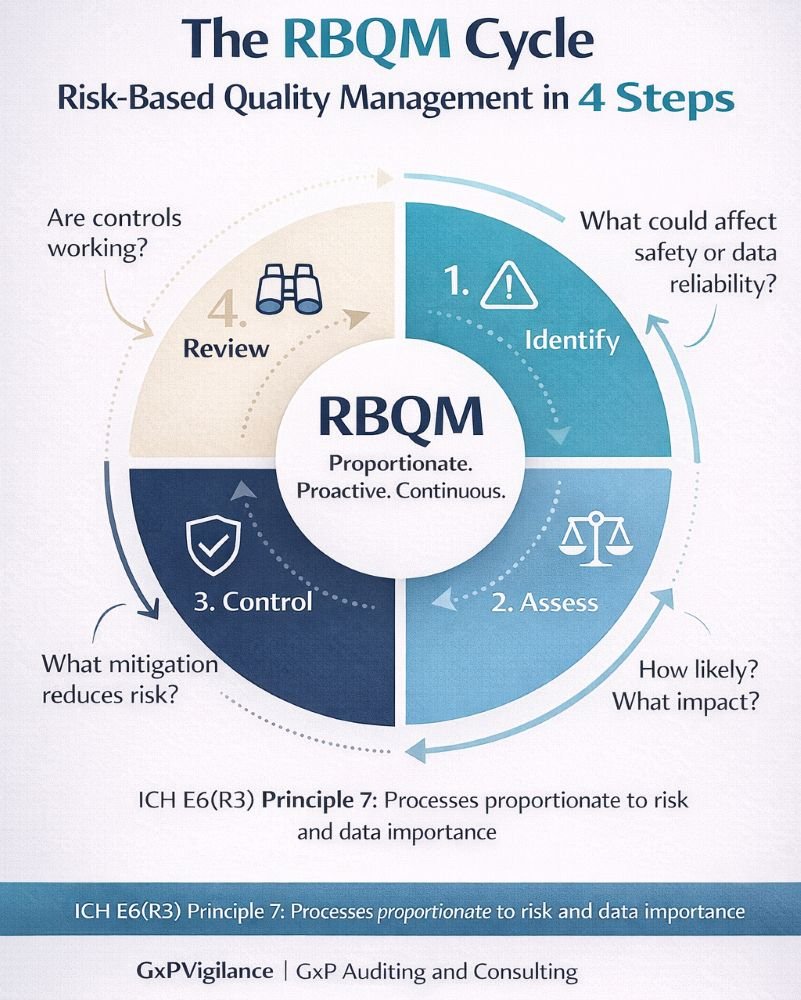
Risk-Based Quality Management in Practice
- Risk Identification: What could go wrong that affects participant safety or data reliability?
- Risk Assessment: How likely is each risk, and what is its potential impact?
- Risk Control: What mitigation strategies reduce the likelihood or impact?
- Risk Review: How do we monitor whether controls work effectively?
Sponsor Oversight: CROs, Vendors, and Delegated Activities
When sponsors delegate activities to CROs or vendors, they must maintain active oversight. This distinction is important during inspections.
Effective oversight mechanisms include:
- Metrics and KPIs tracking vendor performance against agreed standards
- Issue escalation pathways ensuring problems reach sponsor decision-makers
- Audit programs providing independent verification of vendor compliance
Regulators expect evidence of oversight, such as meeting minutes documenting performance reviews, correspondence addressing issues, and escalation records showing sponsor engagement. Sponsors who cannot provide this evidence will struggle to demonstrate oversight.
Aligning QMS With Inspection Readiness and Continuous Improvement
In Australian clinical trials, there is a key difference between inspection readiness and inspection panic. Inspection readiness means the QMS is up to date, staff follow processes, and evidence is always accessible. Inspection panic involves rushing to gather evidence after notification, exposing gaps between documented systems and actual practice.
Building inspection readiness requires:
- Regular internal audits identify issues before inspectors do
- Metrics review tracking QMS performance indicators
- CAPA effectiveness checks verifying that corrective actions achieve intended outcomes
- Feedback loops incorporating lessons learned into system improvements
A sustainable quality culture views audits as opportunities for improvement.
Conclusion: Quality Systems as a Sponsor’s Strategic Asset
- Risk protection, reducing regulatory findings, and trial disruption
- Trial enabler supporting efficient conduct through clear processes
- Confidence builder demonstrating to regulators, ethics committees, and partners that oversight is real
Sponsor accountability cannot be outsourced. Whether using an integrated corporate QMS or relying on CRO systems with hybrid oversight, sponsors must understand, implement, and defend their quality systems.
As ICH E6(R3) takes effect and TGA expectations evolve, the focus on proportionate, risk-based quality systems will increase. Sponsors who treat QMS as a strategic asset, rather than a compliance burden, will be better positioned to conduct trials that protect participants, generate reliable data, and withstand regulatory scrutiny.
Common Questions and Answers
What is a Quality Management System in clinical trials?
A Quality Management System (QMS) is the organised structure of policies, procedures, and processes ensuring consistent trial conduct, controlled documentation, and systematic risk management. In clinical trials, it provides evidence that participant safety and data integrity are protected throughout the trial lifecycle.
Are sponsors required to have a QMS under Australian regulations?
Yes. As of 13 January 2026, the TGA has formally adopted ICH E6(R3), making a systematic Quality Management System a mandatory condition for trials conducted under the CTN and CTA schemes. While the Therapeutic Goods Act 1989 provides the overarching legal framework, the updated TGA Clinical Trial Handbook and Therapeutic Goods Regulations 1990 now explicitly require sponsors to implement a QMS that is “proportionate to the risks of the trial.” We are currently in a 12-month transition period (ending January 2027); however, all new trials must now demonstrate a “Quality by Design” (QbD) approach to ensure participant safety and data reliability from the outset.
Does delegating activities to a CRO transfer sponsor quality responsibilities?
No. ICH GCP E6(R3) Principle 10 explicitly states that sponsors retain overall responsibility for quality and data integrity even when activities are delegated. Sponsors must maintain documented oversight of CRO performance and be prepared to demonstrate this during inspections.
What is the difference between Quality by Design and Risk-Based Quality Management?
Quality by Design (QbD) focuses on building quality into trial design from the outset—identifying Critical-to-Quality factors during protocol development. Risk-Based Quality Management (RBQM) addresses ongoing risk identification, assessment, control, and review throughout trial conduct. Both are complementary and expected under ICH E6(R3).
What evidence do TGA inspectors expect regarding sponsor oversight?
Inspectors look for documented governance structures, vendor qualification files, quality agreements, oversight meeting minutes, performance metrics, issue escalation records, CAPA documentation, and training evidence. The principle “absence of evidence is evidence of absence” applies—if documentation does not exist, inspectors will conclude oversight did not occur.
How often should sponsors review their QMS?
QMS reviews must be risk-proportionate and are no longer strictly limited to an annual calendar event. While formal Management Reviews should still occur at least annually to assess high-level performance, the system must include “active” review triggers, such as: • Significant Quality Issues (SQIs): Any major breach or systemic failure must trigger an immediate QMS sub-review. • Quality Tolerance Limits (QTLs): When trial-specific data trends deviate from predefined acceptable ranges. • Regulatory Updates: Such as the 2026 shift to E6(R3) principles. Internal audits should be scheduled based on the complexity and risk profile of the specific trial portfolio rather than a “one-size-fits-all” timeframe.
What happens if a sponsor lacks an effective QMS during a TGA inspection?
Inspection findings may range from observations requiring corrective action to critical findings affecting trial continuity. Systemic QMS failures can result in trial suspension, data credibility concerns affecting regulatory submissions, and reputational damage with ethics committees and partners. The cost of remediation far exceeds the investment in building effective systems proactively.
How should sponsors select and qualify clinical trial sites?
Sponsors are responsible for selecting investigators and institutions with appropriate qualifications, experience, and resources to conduct the trial properly. ICH GCP E6(R3) Section 3.7 requires sponsors to ensure each investigator is qualified by training and experience and has adequate resources. Site selection should include documented feasibility assessments—ideally conducted independently—evaluating the investigator’s qualifications, site infrastructure, patient population access, regulatory compliance history, and available resources. The sponsor must verify that sites have appropriate facilities, equipment, laboratory capabilities, and trained staff to meet protocol requirements. For investigator-initiated trials (IITs) where the investigator also assumes sponsor responsibilities, institutions must ensure the sponsor-investigator can fulfill both sets of obligations through institutional sponsorship committees that assess risks and oversight requirements. Documentation should include curriculum vitae, GCP training certificates, site evaluation reports, regulatory inspection history, and resource capacity assessments.
What are the essential components of the Trial Master File (TMF), and who is responsible for maintaining it?
Under ICH E6(R3), the TMF has evolved from a collection of “Essential Documents” to a comprehensive set of “Essential Records.” This includes not just PDFs and paper logs, but also metadata, digital audit trails, and communication logs necessary to reconstruct the trial conduct. • Sponsor TMF: Managed by the sponsor (or CRO), containing central documentation (Protocol, IB, Regulatory clearances, Vendor Oversight). • Investigator Site File (ISF): Maintained at the site, containing participant-level data (Consent forms, Source docs, Site-specific logs). • Responsibility: The sponsor retains ultimate accountability for the TMF’s integrity, even if a CRO hosts the eTMF system. Sponsors must ensure they have “continuous and contemporaneous” access to these records. • Australian Retention: Records must be retained for at least 15 years post-trial (per the Australian Code for the Responsible Conduct of Research). For trials involving minors, records must be kept until the participant reaches age 25 or for 15 years, whichever is longer. For ATMPs (Gene/Cell therapies), retention may extend to 30+ years.
What role do quality agreements play in sponsor-CRO relationships?
Quality agreements are formal, documented contracts that clearly define roles, responsibilities, and quality expectations when sponsors delegate trial-related activities to CROs or other service providers. ICH GCP E6(R3) Principle 10.2 mandates that “Agreements should clearly define the roles, activities and responsibilities for the clinical trial and be documented appropriately.” Essential elements of quality agreements include: scope of delegated activities with specific deliverables; quality standards and performance metrics; monitoring and oversight mechanisms; data ownership and access rights; audit rights for sponsor, regulatory authorities, and ethics committees; communication and escalation procedures; CAPA requirements; training and qualification standards; record retention and TMF responsibilities; and terms for subcontracting to third parties. Sponsors must implement documented oversight mechanisms to verify CRO compliance with quality agreements, including regular performance reviews using outcome-based metrics (data quality indicators, safety reporting timeliness, protocol deviation rates) rather than just activity measures. ICH E6(R3) Section 3.9 “Sponsor Oversight” requires appropriate oversight of all trial-related activities carried out by service providers, including those subcontracted by the primary CRO. Inspection findings consistently identify inadequate quality agreements and insufficient documented oversight as common sponsor deficiencies. The sponsor’s oversight system should include vendor qualification processes, ongoing performance monitoring, documented oversight meeting minutes, trending analysis of quality metrics, and evidence of corrective action when performance gaps are identified.
References:
- Therapeutic Goods Administration (TGA) – Good Clinical Practice (GCP) Inspection Program 2023-2024 (Australian Government Department of Health and Aged Care)
- International Council for Harmonisation (ICH) – ICH E6(R3) Guideline for Good Clinical Practice (Final Version, Adopted 6 January 2025)
- Therapeutic Goods Administration (TGA) – Australian Clinical Trial Handbook (Australian Government Department of Health and Aged Care, Version 3, October 2024)
- National Health and Medical Research Council (NHMRC) – National Statement on Ethical Conduct in Human Research 2025 (NHMRC, Australian Research Council, Universities Australia)
- Australian Commission on Safety and Quality in Health Care – National Clinical Trials Governance Framework (May 2022, Australian Government)
- Medicines and Healthcare products Regulatory Agency (MHRA) – Sponsor Oversight of Clinical Trials (MHRA Inspectorate Blog Series, Parts 1 and 2, July-September 2018, UK Government)
- U.S. Food and Drug Administration (FDA) – Sponsor Oversight in Clinical Trials: Session Presentations (FDA Clinical Trial Transparency Workshop, February 2024, U.S. Department of Health and Human Services)
Therapeutic Goods Administration (TGA) – Good Clinical Practice (GCP) Inspection Program (Australian Government Department of Health and Aged Care)
Therapeutic Goods Administration (TGA) – Good Clinical Practice (GCP) Inspection Program 2023-2024 (Metrics Report, Australian Government Department of Health and Aged Care)
Disclaimer
This article is provided for educational and informational purposes only. It is intended to support general understanding of regulatory concepts and good practice and does not constitute legal, regulatory, or professional advice.
Regulatory requirements, inspection expectations, and system obligations may vary based on jurisdiction, study design, technology, and organisational context. As such, the information presented here should not be relied upon as a substitute for project-specific assessment, validation, or regulatory decision-making.
For guidance tailored to your organisation, systems, or clinical programme, we recommend speaking directly with us or engaging another suitably qualified subject matter expert (SME) to assess your specific needs and risk profile.
GxPVigilance can help ensure inspection-ready compliance.




