The GCP auditor in Australia is pivotal yet often misunderstood. This role demands more than procedural checks; it is fundamental in safeguarding clinical trial integrity, balancing evolving regulations, patient safety, and productive collaboration.
GCP auditors are the operational conscience of Australia’s clinical research, directly protecting patient safety and research quality. Auditors who excel proactively find vulnerabilities, support compliance, and ensure timely patient access to treatments. When they fall short, patients are at risk, and sponsors face costly delays.
Australia’s clinical trial sector has grown, attracting international sponsors with competitive R&D tax incentives and a reputation for high-quality data. This growth has increased demand for GCP auditors who know local TGA requirements and the international frameworks sponsors require. The profession sits at a critical intersection: regulatory compliance, patient protection, and commercial viability all depend on auditors’ performance.
This article examines what makes a GCP auditor in Australia effective—the qualifications, regulatory knowledge, experience, personality traits, and continuous learning that set competent auditors apart.
What Makes GCP Auditing Different
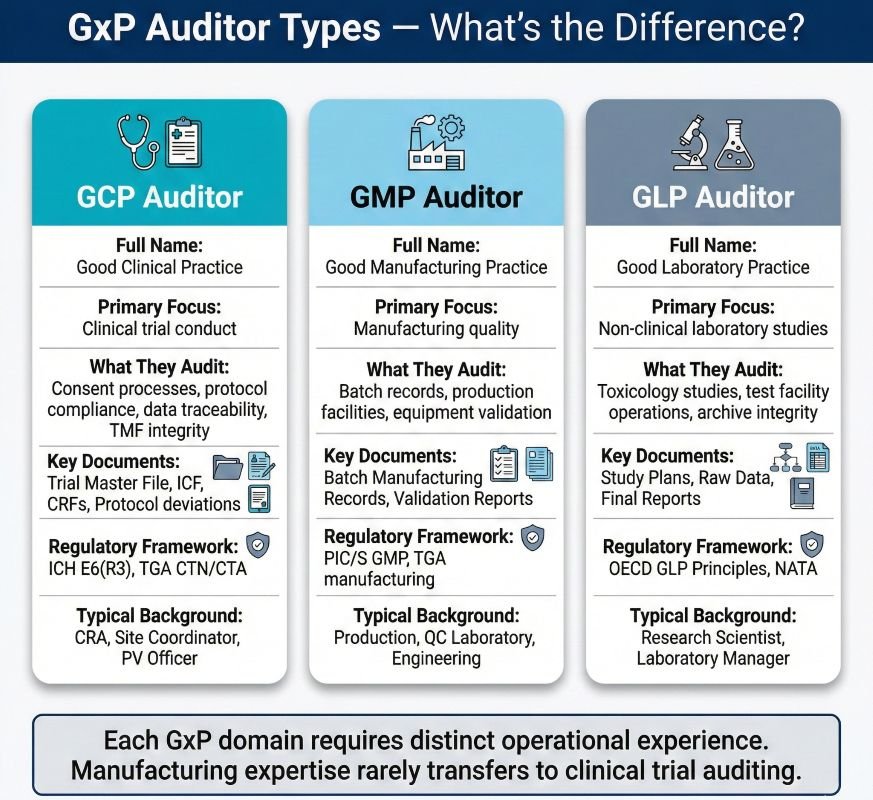
GCP (Good Clinical Practice) auditing differs from other GxP (Good Practice) disciplines. GMP (Good Manufacturing Practice) auditors focus on manufacturing controls. GLP (Good Laboratory Practice) auditors assess non-clinical laboratory studies. The GCP auditor evaluates the conduct of clinical trials: informed consent processes, protocol adherence, investigational product management, and data integrity across complex multi-site operations.
The scope covers the full clinical supply chain. Sponsors maintain oversight. CROs carry out monitoring. Investigator sites recruit and treat participants. Hospital pharmacies dispense trial medications. Central laboratories analyse samples. Each element demands different technical knowledge and assessment.
Key distinction: Process auditing at this level needs formal training and recognised qualifications. A GCP auditor must interpret regulations, not apply them mechanically. ICH E6(R3) took effect in Australia in January 2026. Sponsors now must use risk-based quality management. Auditors assess if organisations have identified Critical-to-Quality factors and applied proportionate controls, not just if procedures exist.
Regulatory Foundations: Local and Global
A GCP auditor in Australia operates within overlapping regulatory frameworks: the TGA adopts ICH GCP guidelines and enforces compliance through inspections; the NHMRC sets ethical conduct requirements through the National Statement; and the Therapeutic Goods Act 1989 provides the legal foundation.
Auditors must also understand international expectations. Multinational sponsors expect Australian sites to simultaneously satisfy FDA, EMA, and other regulatory authority standards. This creates practical complexity: an auditor evaluating a Sydney-based CRO running both domestic and international trials must assess compliance with multiple overlapping frameworks.
| Framework / Regulation | Jurisdiction | 2026 Auditor “Critical Focus” |
|---|---|---|
ICH E6(R3) | Global / TGA | The Transition Rule: TGA adopted R3 on Jan 13, 2026. Auditors must verify if the site is in the “Transition Period” (ending Jan 2027) and if Quality by Design (QbD) principles are documented. |
NCTGF (Governance Framework) | Australia | Hospital Accreditation: Mandatory since 2025. Auditors must check if trials are integrated into the hospital’s NSQHS Standards and overseen by the board, not just a research office. |
National Statement (2025/26) | Australia | Precedence: TGA confirms the National Statement takes precedence over ICH GCP in ethics. Focus on updated consent for AI and vulnerable groups. |
Therapeutic Goods Act 1989 | Australia | CTN/CTA Maintenance: Proper use of the “National One Stop Shop” portal and compliance with unapproved product supply (SAS / Section 19A). |
Privacy Act & APPs | Australia | Data Breach Readiness: Evidence of a Notifiable Data Breach (NDB) plan and strict control over cross-border data flows for global sponsors. |
FDA 21 CFR Part 11 | USA (FDA) | Diversity Action Plans: FDA now requires “Diversity Action Plans.” Auditors must verify that the site has strategies to meet the sponsor’s US-mandated demographic targets. |
EU CTR (536/2014) | Europe (EMA) | CTIS Transparency: All data bound for Europe must be “redaction-ready” for the Clinical Trials Information System. Focus on strict 15-day reporting windows. |
UK Clinical Trial Regs (2026) | UK (MHRA) | New Terminology: Effective April 28, 2026. “Subject” → Participant; “Site” → Trial Location. 12-month mandatory results publication. |
ISO 14155:2020 | Global (Devices) | Device GCP: Verification of “Device Deficiencies” vs. SAEs and clinical evaluation reports for MedTech-specific trials. |
NHMRC AI Ethics Guide (2025) | Australia | Algorithmic Bias: If the trial uses AI (e.g., for imaging or screening), auditors must see evidence of Bias Mitigation and Human-in-the-loop oversight. |
PIC/S Guide to GMP | Global (IMP) | Cold Chain & Labeling: Verification of “Release for Supply” and TGO 110 compliance for biologicals/vaccines. |
Gene Technology Act 2000 | Australia | OGTR Licensing: Mandatory for GMO trials (CAR-T, mRNA vaccines). Audit of the Institutional Biosafety Committee (IBC) approvals. |
The auditor’s value lies in translating these requirements into practical operational guidance. A finding that simply cites a regulation without explaining the underlying risk or providing a defensible remediation pathway adds limited value to the auditee.
Experience, Capability, and Professional Maturity
GCP auditors rarely begin their careers in auditing. Most start in operational roles—such as Clinical Research Associate, pharmacovigilance officer, hospital pharmacist, or laboratory scientist—then move into quality assurance and later to auditing. This operational background gives them the context needed to turn audit findings into actionable intelligence.
Typical experience requirements:
- Entry-level QA roles: 2-3 years of operational experience
- Independent auditor positions: 5+ years in quality assurance, including 3+ years conducting audits
- Senior lead auditor roles: 10+ years combined GxP experience, 5+ years leading audit programmes
Career progression typically unfolds across three phases:
- Early-stage (0-3 years): Supporting internal audits, managing CAPA documentation, and observing senior auditors during site visits.
- Mid-career (3-7 years): Owning full audit cycles, specialising in specific areas (site audits, vendor qualification, GVP).
- Senior progression (7+ years): Shaping annual audit programmes, integrating findings with clinical operations, co-owning inspection readiness
The Australian market accepts several qualification paths. ISO 9001 Lead Auditor credentials give a basic understanding of audit methods. Specialised GxP training by organisations such as SeerPharma and ARCS builds pharmaceutical skills. More often, auditors get ISO 42001 certification to check AI systems as automated tools enter GxP work.
- Curiosity: The compulsion to keep asking “why?” until root causes emerge
- Resilience: Maintaining objectivity despite pushback or organisational pressure. Independence: Drawing conclusions solely from objective evidence, not influenced by relationships or politics.
- Ethical judgement: Reporting critical findings, even when doing so may create discomfort.
- Diplomatic directness: Providing constructive, clear feedback on difficult issues without diluting key points.
ISO 19011 names thirteen key behavioural competencies for auditors. These include being ethical, open-minded, observant, perceptive, versatile, tenacious, and decisive.
The GCP auditor in Australia must balance these: be firm enough to classify real risks, yet flexible enough to know there are many ways to meet regulatory requirements.
Common misconception: Effective auditors are not adversarial “gotcha” inspectors.
The modern approach emphasises partnership. Auditors identify systemic vulnerabilities and guide sustainable improvement. Their goal is not simply documenting failures. Organisations that treat auditors as enemies tend to have poor disclosure. Those treating them as trusted advisors benefit from genuine quality improvement.
Continuous Learning in a Changing Landscape
The regulatory environment evolves continuously. In 2024 alone, the FDA published 42 new or revised guidance documents relevant to pharmaceutical operations. TGA regularly updates its expectations, and ICH E6(R3) introduced substantial changes to risk-based quality management requirements. An auditor who stops learning quickly becomes outdated.
Regulatory intelligence forms the foundation of professional currency. Experienced auditors subscribe to official channels, including TGA guidance updates, FDA warning letters, EMA questions and answers, and ICH guideline revisions. Many use commercial regulatory intelligence services or AI-powered monitoring tools that flag relevant changes based on their professional interests. This systematic approach prevents auditors from being surprised by requirements their auditees have already implemented.

Effective continuous learning strategies:
- Subscribe to regulatory intelligence services covering TGA, FDA, EMA, and ICH updates.
- Complete transition training for major guideline revisions (currently ICH E6(R3))
- Attend industry conferences to learn about practical implementation case studies.
- Participate in professional networks sharing de-identified audit experiences.
- Analyse regulatory inspection findings to identify emerging themes
- Develop competency in Computerised System Validation and data integrity assessment.
- Build AI literacy to evaluate automated tools entering GxP operations.
The AI dimension now requires special attention. Sponsors use machine learning for patient recruitment, adverse event detection, and adaptive trial design. Auditors must verify that these systems are properly validated. Traditional methods fall short for models that learn from new data. Auditors need new frameworks for evaluating transparency, training data, and oversight controls.
Forward-thinking auditors use AI tools too. Central monitoring platforms analyse clinical data to find outlier sites or suspicious patterns before audits. Natural language processing tools scan thousands of documents to flag missing signatures or inconsistent dates. There is professional debate about how much AI is too much; each auditor must decide how to avoid over-reliance on these tools.
Conclusion: Why Great GCP Auditors Matter
The GCP auditor in Australia serves a function that extends beyond compliance verification. Effective auditors protect patients by identifying trial conduct vulnerabilities before those vulnerabilities cause harm. They protect sponsors by ensuring inspection readiness before the TGA arrives unannounced. They strengthen Australia’s clinical research reputation by maintaining the standards that attract international trial investment.
The profession requires significant investment: years in operational roles before switching to audits, ongoing qualification maintenance, and ongoing regulatory intelligence gathering. Increasingly, auditors also need technical skills in AI governance. Organisations that understand this—offering resources, professional development, and real partnership—create quality systems that pass any regulatory test.
For those considering this career pathway, the threshold question is straightforward: Can you maintain curiosity, independence, and ethical commitment over a career that will frequently place you in conflict with operational.
If so, a career in this field provides an opportunity to contribute to patient safety while developing expertise applicable across pharmaceutical, biotechnology, and clinical research sectors.
Common Questions and Answers
What qualifications do I need to become a GCP auditor in Australia?
Most auditors hold a life-science or nursing degree plus clinical research experience; ISO 9001/GCP auditing courses and GxP training significantly strengthen your profile.
How long does it take to become an effective GCP auditor?
Plan on 5–7 years: a few years in operational roles (e.g., CRC/CRA/QA) then several years of supervised auditing, with lead auditor roles often requiring 10+ years’ GxP experience.
What is the difference between a GCP auditor and a TGA inspector?
GCP auditors work for sponsors, CROs, or consultancies to identify and fix GCP gaps; TGA inspectors are regulators who perform official inspections and can issue findings or enforcement actions.
How has ICH E6(R3) changed GCP auditing requirements in Australia?
E6(R3) (being adopted by TGA with a transition period) pushes auditors to test risk-based quality management, Critical-to-Quality factors, and ongoing oversight, not just SOP compliance.
Do GCP auditors need to understand AI and digital systems?
Yes—auditors increasingly assess validation, data integrity, and governance of eTMF, CTMS, RBM tools and AI/ML systems used for monitoring, safety, and data review.
What is the average salary of a GCP auditor in Australia?
In cities like Melbourne, GCP auditors earn around AUD 110k on average, with roughly AUD 60k–160k+ depending on experience, seniority, and organisation type.
How do I transition from CRA or study coordinator to a GCP auditor role?
Build 2–4 years’ strong GCP experience in site or monitoring roles, then move into QA by supporting audits, drafting CAPAs, and adding formal GCP, auditing, or Quality Auditing qualifications.
Do I need formal GCP certification or auditor training to become a GCP auditor?
You’ll usually need current ICH-GCP training plus evidence of auditing skills gained through on-the-job mentoring and courses such as ISO 9001 internal or lead auditor programs.
What types of organisations employ GCP auditors in Australia?
GCP auditors are hired by pharma and biotech sponsors, CROs, hospital and university research offices, and independent consultancies providing GCP audit and inspection-readiness services.
Which courses and certifications are most useful if I want to become a GCP auditor?
Look for Australia-adapted ICH-GCP courses, practical GCP or clinical auditing programs, ISO 9001 auditor training, and diplomas in Quality Auditing or Quality Management.
References:
International Council for Harmonisation (ICH) – ICH E6(R3) Guideline for Good Clinical Practice (Final Version, Adopted 6 January 2025)
Therapeutic Goods Administration (TGA) – Good Clinical Practice (GCP) Inspection Program 2023-2024 (Australian Government Department of Health and Aged Care, Version 1.0, March 2025)
National Health and Medical Research Council (NHMRC) – National Statement on Ethical Conduct in Human Research 2023 (NHMRC, Australian Research Council, Universities Australia, Effective 1 January 2024)
National Health and Medical Research Council (NHMRC), Australian Research Council (ARC), Universities Australia – Australian Code for the Responsible Conduct of Research 2018 (NHMRC, ARC, Universities Australia, 2018)
Therapeutic Goods Administration (TGA) – Australian Clinical Trial Handbook (Australian Government Department of Health and Aged Care, Current version available online with regular updates)
Disclaimer
This article is provided for educational and informational purposes only. It is intended to support general understanding of regulatory concepts and good practice and does not constitute legal, regulatory, or professional advice.
Regulatory requirements, inspection expectations, and system obligations may vary based on jurisdiction, study design, technology, and organisational context. As such, the information presented here should not be relied upon as a substitute for project-specific assessment, validation, or regulatory decision-making.
For guidance tailored to your organisation, systems, or clinical programme, we recommend speaking directly with us or engaging another suitably qualified subject matter expert (SME) to assess your specific needs and risk profile.




