Introduction
Remember the last time you waited months for a clinical trial to start? Site approvals stalled. Ethics committees requested clarification. The TGA acknowledgement sat in limbo. Your investigational product gathered dust while timelines slipped and budgets strained.
Australia’s clinical trial system doesn’t need to work this way. The Therapeutic Goods Administration (TGA) provides two distinct pathways for supplying unapproved therapeutic goods in clinical trials—the Clinical Trial Notification (CTN) scheme and the Clinical Trial Approval (CTA) scheme. Each serves different purposes, carries different timelines, and requires different levels of TGA involvement before your trial starts recruiting.
For pharmaceutical and biotech sponsors operating in Australia’s regulated healthcare environment, understanding these pathways helps prevent costly delays. This guide explains how the TGA regulates clinical trials, what ethics committees require, when institutional authorisation becomes mandatory, and how to structure your submission so approvals happen in parallel rather than sequence.
Overview: Australia’s Dual Clinical Trial Pathway System
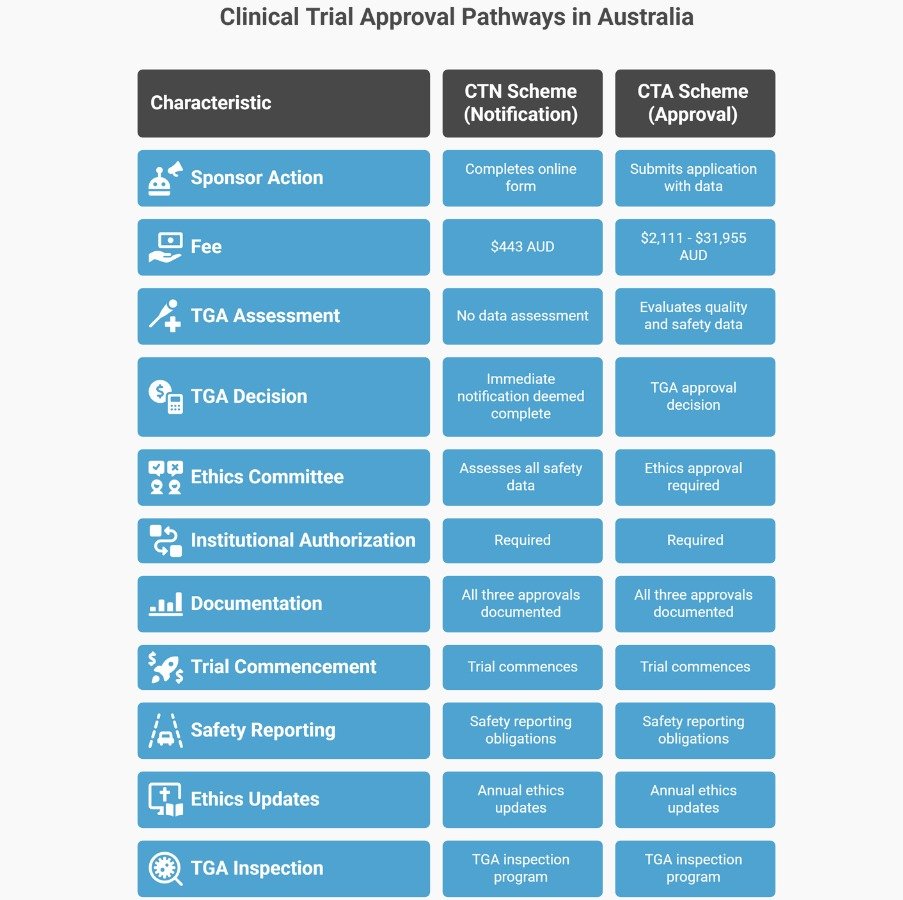
The TGA allows unapproved therapeutic goods to be supplied in clinical trials under two regulatory schemes mandated by the Therapeutic Goods Act 1989 and Therapeutic Goods Regulations 1990. The distinction between these schemes determines how much data the TGA reviews before your trial commences.
The fundamental split:
- CTN scheme (notification) — The TGA doesn’t assess clinical data before the trial starts. The sponsor notifies intent, pays the fee, and may lawfully supply the goods immediately. The ethics committee (called the Human Research Ethics Committee, or HREC, in Australia) is responsible for assessing scientific validity, risk-benefit balance, and ethical acceptability.
- CTA scheme (approval) — The TGA evaluates quality, preclinical, and early clinical safety data before authorising supply. The sponsor submits a formal application with supporting evidence. The TGA conducts a scientific assessment that typically involves multiple evaluation rounds. Only after TGA approval—and ethics committee approval—can the trial commence.
When Each Scheme Applies
The sponsor decides which scheme to use by considering product risk, available data, ethics committee capacity, and institutional requirements. However, CTA is mandatory for specific Class 4 biologicals, regardless of the sponsor’s preference.
Some ethics committees require TGA acknowledgement before beginning their review, even under CTN. Others accept parallel processing. Check institutional policies early—the TGA will accept CTN submissions before ethics approval is finalised, but the trial cannot start until all approvals (TGA notification, ethics approval, institutional authorisation) are documented.
The CTN Scheme: Notification Without Assessment
How It Works
The CTN scheme is a notification process. The sponsor completes an online form through TGA Business Services, pays the application fee ($443 AUD for medicines or biologicals as of 2025), and receives immediate acknowledgement. The trial is deemed notified once the online form is submitted and the fee is paid.
No waiting for TGA review. The sponsor may lawfully supply the investigational product immediately—assuming ethics approval and institutional authorisation are already in place. If you’re notifying before those approvals finalise, you’re responsible for ensuring everything aligns before participants enrol.
The TGA doesn’t evaluate your investigational brochure, preclinical data, or trial protocol at the time of notification. That responsibility sits with the ethics committee, which must assess:
- Scientific validity of trial design
- Balance of risk versus potential benefit
- Overall ethical acceptability
- Adequacy of informed consent processes
When the TGA Can Intervene
Even though CTN doesn’t include an upfront assessment, the TGA retains authority. If the TGA becomes aware that conducting or continuing your trial would be contrary to the public interest—particularly if an unacceptable risk of death, serious illness, or serious injury exists—the TGA can direct the trial not to proceed. At that point, the goods lose their exemption from the Australian Register of Therapeutic Goods (ARTG), and supply becomes unlawful.
The TGA may also request additional information if the trial raises concerns. You’re required to provide details about supply, handling, monitoring, and results if asked.
Note: All these documents can be reviewed during the TGA GCP Inspection.
Documentation Requirements
The online CTN form captures:
- Sponsor details and Australian contact information
- Protocol number and trial description (250-2,500 characters)
- Trial type, therapeutic area, and participant numbers
- Investigational product details: dosage form, route of administration, indication, and GMP license/clearance
- Site details and preceding trial information
- Declaration confirming sponsor responsibility
Critical: If you add new sites to an existing CTN trial, that’s a variation requiring a new fee ($443 AUD per site notification). If you add a new therapeutic good or create a distinct product by changing the notified good, that’s also a fee-triggering variation.
The CTA Scheme: TGA Assessment Before Supply
How It Works
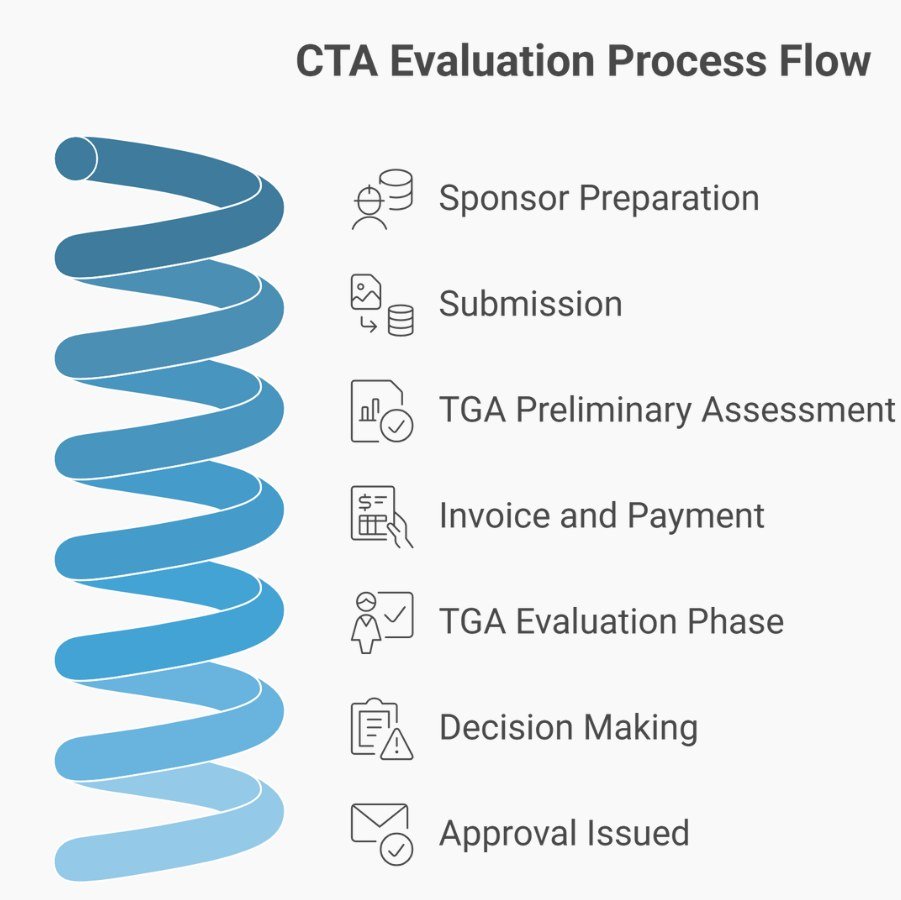
CTA involves a formal application to the TGA for approval to supply unapproved therapeutic goods in a clinical trial. The sponsor submits:
- Part 1: CTA application form with supporting data (quality, preclinical, early clinical safety)
- Part 2: Trial commencement notification (submitted within 28 days of commencing supply at each site)
The TGA conducts a preliminary assessment to ensure sufficient data exists for evaluation. If critical data is missing, the TGA requests it before issuing an invoice. Evaluation begins after payment. The TGA generally completes two rounds of assessment and one round of information requests, though additional rounds may be needed.
Evaluation covers:
- Quality of the therapeutic goods
- Safety of the therapeutic goods
- Compliance with applicable Therapeutic Goods Orders (TGOs)
- Compliance with international guidelines (ICH, FDA, EMA)
- Labelling and traceability
A TGA senior medical officer reviews evaluation reports and recommendations, considering the overall risk-benefit profile. The decision-maker may seek advice from the TGA’s statutory advisory committees. The TGA informs the sponsor whether the trial is approved.
CTA Variations
If you need to change the therapeutic good or any aspect evaluated by the TGA in your original submission, you’ll likely need a variation requiring additional evaluation and a fee payment. Changes predicted to affect product quality, safety, or the trial’s risk-benefit profile trigger assessment. Examples include:
- Significant manufacturing process changes
- Different intended patient group
- Route of administration changes
- Container modifications
Changes not evaluated in the original submission or with no predicted effect on the good or trial safety won’t be assessed by the TGA, but all changes must be communicated to and approved by the ethics committee before implementation.
Fees and Timelines
CTA fees vary by product type and evaluation timeline [2025]:
- Medicines CTA (30-day evaluation): $2,111 AUD
- Medicines CTA (50-day evaluation): $26,240 AUD
- Biologicals CTA: $31,955 AUD
- CTA variations: $580 – $8,718 AUD depending on product and evaluation type
Timeline reality: The TGA doesn’t publish standard review times for CTN or CTA. Contact the TGA for specific timeline inquiries. Most sponsors report CTA taking several months when evaluation rounds and information requests are factored in.
Ethics Committee Requirements: Non-Negotiable for Both Schemes
Regardless of which TGA scheme you choose, every clinical trial involving a risk level greater than low requires ethics committee approval. The National Health and Medical Research Council (NHMRC) publishes the National Statement on Ethical Conduct in Human Research 2023, which governs how ethics committees operate.
Ethics Committee Composition
Australian ethics committees must include at a minimum:
- A chairperson with suitable experience
- Two lay members with no paid affiliation to the institution
- One person with knowledge of professional care, counselling, or treatment
- One person performing pastoral care
- One qualified lawyer
- Two people with current research experience relevant to proposals being reviewed
At least one-third of participants at each meeting must be external to the institution. Committees reviewing research on Aboriginal and Torres Strait Islander peoples require members with relevant cultural knowledge.
What Ethics Committees Review
The ethics committee assesses whether your trial protocol complies with:
- National Statement on Ethical Conduct in Human Research 2023
- Declaration of Helsinki
- ICH GCP (Good Clinical Practice) guideline as annotated by the TGA
- TGA requirements, including the Australian Clinical Trial Handbook
- Relevant Commonwealth, state, and territory laws
The committee also evaluates privacy protection, ensuring alignment with federal and state privacy legislation.
National Mutual Acceptance (NMA) Scheme
The NMA scheme supports a single ethical review for multicenter research conducted in publicly funded health services. All Australian state and territory-certified public health organisations participate. For ethics reviews to be accepted under NMA, the reviewing committee must be certified under the NHMRC National Certification Scheme.
This prevents the nightmare scenario where five hospitals in different jurisdictions each require separate ethics applications for the same protocol. One certified committee reviews. Other sites accept that review. However, each site still conducts its own site-specific assessment (SSA) and issues its own institutional authorisation.
Site-Specific Assessment and Institutional Authorisation
Ethics approval doesn’t mean you can start the trial. Each participating institution must complete a site-specific assessment evaluating:
- Whether the project is suitable for the site
- Whether the institution has the capacity to conduct the research
- Local resource availability (staff, equipment, facilities)
- Relevant local considerations that ethics review wouldn’t capture
Critical timing: Ethics approval must be obtained and submitted to the research governance officer (RGO) at each institution before institutional authorisation is granted. The SSA and ethics review may occur in parallel, but authorisation cannot be issued until ethics approval is documented.
Jurisdiction-Specific Platforms
Different Australian jurisdictions use different systems for SSA submissions:
- New South Wales and Australian Capital Territory: Research Ethics and Governance Information System (REGIS)
- Queensland, Victoria, and Mater Research: Ethical Review Manager (ERM) website
- South Australia: Research GEMS system
- Northern Territory, Tasmania, Western Australia: Contact the ethics committee for local requirements
If you’re running a multicenter trial across jurisdictions, you’ll navigate multiple platforms. Plan accordingly.
Safety Reporting: What Triggers Reporting Obligations
Australian clinical trial safety reporting follows the TGA’s Clinical Trials Handbook and NHMRC Safety Monitoring and Reporting in Clinical Trials Involving Therapeutic Goods guidance (2016).
Investigator Responsibilities
The investigator must report to the sponsor within 24 hours:
- All serious adverse events (SAEs)
- All urgent safety measures (USMs) instigated by the site
The investigator must report to the institution within 72 hours:
- USMs instigated by the investigator or site
- Suspected unexpected serious adverse reactions (SUSARs) arising from the local site
- Information from the sponsor that may affect continued ethical acceptability or require protocol amendments
Sponsor Responsibilities
The sponsor must provide expedited reporting (Pharmacovigilance Responsibilities) of SUSARs to the TGA within:
- 7 calendar days (with 8-day follow-up) for Australian SUSARs that are fatal or life-threatening
- 15 calendar days for all other Australian SUSARs
Significant safety issues (SSIs) that meet the definition of USM must be reported to the TGA, ethics committee, and investigators within 72 hours. All other SSIs must be reported within 15 calendar days.
The TGA strongly recommends contacting them within 24 hours of a USM being taken, followed by written notification within 72 hours.
Annual Reporting
Individual adverse event reports to ethics committees are no longer required. Instead, sponsors must provide the ethics committee with an updated investigator’s brochure at least annually, demonstrating appropriate safety monitoring and depicting the evolving safety profile clearly.
Implications for Practitioners: What This Means for Your Trial
For sponsors choosing between CTN and CTA:
- If your ethics committee has the scientific expertise to assess your product’s safety without TGA input, CTN may be faster
- If your product is novel, high-risk, or the ethics committee requests TGA review, CTA provides that assurance
- CTN costs $443; CTA costs $2,111-$31,955 depending on product and timeline
- CTN allows immediate supply after notification + approvals; CTA requires TGA evaluation completion first
For multicenter trials:
- Single ethics review through NMA prevents duplicate ethics applications across sites
- Each site still requires separate institutional authorisation after SSA
- Budget time for navigating multiple jurisdiction-specific platforms (REGIS, ERM, Research GEMS)
- Don’t assume parallel processing—some sites require sequential approvals
For safety reporting:
- Build 24-hour investigator-to-sponsor reporting into your site training
- Ensure 72-hour sponsor-to-TGA capability for USMs (including weekends)
- Annual investigator brochure updates aren’t optional—ethics committees expect them
For trial commencement:
- You need three green lights before enrolling participants: TGA authorisation (CTN notification or CTA approval), ethics committee approval, and institutional authorisation at each site
- The TGA doesn’t prohibit submitting CTN before other approvals finalise, but you remain responsible for ensuring alignment before starting
Frequently Asked Questions
Can I start my CTN trial immediately after submitting the online form?
Technically, the trial is deemed notified as soon as the online form is submitted and the fee is paid. However, you still need ethics committee approval and institutional authorization at every site before enrolling participants. The TGA notification doesn’t replace those requirements—it runs parallel to them. Some sponsors submit CTN early while finalizing ethics and institutional approvals, but the trial cannot commence until all three are documented.
What happens if my ethics committee says they need TGA review but I’ve already submitted a CTN?
The sponsor controls scheme selection, but practical realities matter. If your ethics committee determines they lack the scientific expertise to assess your product’s safety without TGA input, you’ll likely need to withdraw the CTN and submit a CTA application. This isn’t common, but it does occur with novel products or complex safety profiles. Consulting your chosen ethics committee before submitting can prevent this scenario.
Do I need separate ethics approvals from each hospital in a multicenter trial?
Not necessarily. The National Mutual Acceptance (NMA) scheme enables single ethics review for multicenter research across publicly funded Australian health services. One certified ethics committee reviews the protocol. Other participating sites accept that review and focus on site-specific assessment and institutional authorization. However, each site issues its own authorization separately after evaluating local suitability and capacity.
How long does CTA evaluation actually take?
The TGA doesn’t publish standard timelines, and duration varies based on product complexity, data quality, and whether additional information requests are needed. Sponsors typically report several months from submission to approval, factoring in preliminary assessment, evaluation rounds, information requests, and decision-making. For specific timeline estimates, contact clinical.trials@health.gov.au with your product details.
If I’m running a teletrial with satellite sites, do the satellite sites need separate institutional authorization?
Yes. Even in teletrials where the principal investigator (PI) at the primary site maintains overall responsibility, each satellite site must complete a site-specific assessment and receive institutional authorization from its research governance officer before trial-related activities can occur there. The PI ensures all documentation is filed appropriately, but the satellite site RGO issues authorization separately.
What counts as a “variation” that triggers new fees under CTN?
Adding a new site to an existing CTN trial incurs a variation fee ($443 AUD per site). Adding a new therapeutic good to a previously notified trial also triggers a fee. Changing the notified therapeutic good in a way that creates a separate and distinct product requires a new notification with associated fees. Minor administrative updates to trial details (contact information, expected completion dates) can be updated through the online form without fees, but substantive changes to goods or sites generate charges.
Conclusion: Building Compliant Trials in Australia’s Regulatory Environment
Australia’s clinical trial system balances two competing needs: enabling access to experimental therapies and protecting participants from unacceptable risk. The CTN and CTA schemes reflect this balance—CTN trusts ethics committees to assess product safety when TGA review isn’t necessary; CTA provides TGA evaluation when product risk or novelty warrants it.
Understanding which scheme fits your trial prevents unnecessary delays. More importantly, understanding how TGA authorisation, ethics approval, and institutional authorisation interact prevents the sequential approval nightmare where each process waits for the previous one to complete.
The TGA reformed Australia’s clinical trial framework in recent years, specifically to reduce start-up timelines. Taking advantage of those reforms requires intentional design: parallel submissions where allowed, early consultation with ethics committees and sites, and documentation that meets TGA, NHMRC, and institutional standards simultaneously.
Explore our GCP Audit Services → for independent compliance assessment, or contact us to discuss your trial start-up challenges.
References and Further Reading
Primary Regulatory Documents
- Therapeutic Goods Act 1989
- Therapeutic Goods Regulations 1990
- Australian Clinical Trial Handbook: Guidance on Conducting Clinical Trials in Australia Using ‘Unapproved’ Therapeutic Goods – Therapeutic Goods Administration, October 2024
Ethics and Governance Standards
- National Statement on Ethical Conduct in Human Research 2023 – National Health and Medical Research Council
- National Standard Operating Procedures for Clinical Trials, including Teletrials, in Australia – Department of Health and Aged Care, February 2021
- ICH Guideline for Good Clinical Practice, Annotated with TGA Comments (AU-ICH-GCP) – International Council for Harmonisation and Therapeutic Goods Administration
Safety Reporting
- Safety Monitoring and Reporting in Clinical Trials Involving Therapeutic Goods – National Health and Medical Research Council, November 2016
- Preparing for Good Clinical Practice (GCP) Inspections – Therapeutic Goods Administration
Disclaimer
This article provides educational information on Australia’s clinical trial regulatory framework, based on publicly available TGA guidance, NHMRC standards, and applicable legislation, as of November 2025. While the author holds relevant professional qualifications and regulatory experience, this content does not constitute legal, regulatory, or professional advice specific to your circumstances.
Regulatory requirements change frequently. The Therapeutic Goods Administration (TGA), National Health and Medical Research Council (NHMRC), and state/territory health authorities may update guidance, fees, processes, and compliance expectations without notice. Always verify current requirements directly with the TGA and consult with qualified regulatory affairs professionals, legal counsel, or compliance experts before making decisions that affect your clinical trial operations.




