QPPVA and Pharmacovigilance Roles in Australia matter because a single safety report can change everything for a sponsor. This 2025 guide explains who leads pharmacovigilance in Australia, how the QPPVA and contact person roles work in practice, and what sponsors must do to stay inspection-ready across AU, NZ, and the EU.
Across jurisdictions, the titles differ — QPPVA, Contact Person, or EU-QPPV — but the expectation is the same: to safeguard patient safety and maintain the integrity of your pharmacovigilance system.
This article clarifies how these roles function across three major regulatory frameworks (TGA, Medsafe, and EMA), what each expects of sponsors, and how to build a system that remains inspection-ready every day — not just audit-ready once a year.
Australia: QPPVA and Pharmacovigilance Roles in Australia — What the TGA Requires
The Legal Foundation: What the TGA Actually Requires
The Therapeutic Goods Act 1989 establishes the legal foundation for Australian pharmacovigilance. Under this framework, the TGA mandates that medicine sponsors nominate an “Australian pharmacovigilance contact person.” This notification must occur within 15 calendar days of your first ARTG entry or whenever contact details change.
In practice, while regulations specify a “contact person,” the pharmaceutical industry commonly uses the title “Qualified Person for Pharmacovigilance in Australia” (QPPVA), which often aligns with the role of a contact person. In Australia, each role can be filled by a different person, but both designations are required. This approach reflects global best practices influenced by EU standards. As a result, the TGA’s Pharmacovigilance Inspection Program (PVIP) assesses this role against evolving expectations.
Beyond Contact: What “QPPVA” Really Means in Practice
The adoption of the QPPVA title indicates responsibilities that extend well beyond basic liaison duties. This individual oversees the entire Australian PV system, ensuring robust processes for collecting, assessing, and reporting safety information. They also maintain ongoing inspection readiness, which is essential given the TGA’s ability to audit at any time.
This distinction is critical during inspections. Appointing a junior-level contact without sufficient authority increases compliance risk. Inspectors expect a representative who can speak authoritatively about system architecture, demonstrate decision-making authority, and show comprehensive oversight of safety obligations.
Core Responsibilities in Australia — QPPVA and Pharmacovigilance Roles
The Australian QPPVA keeps abreast of several critical, time-bound obligations:
- Serious Adverse Reactions: Sponsors must report all serious reactions in Australia to the TGA within 15 calendar days of becoming aware of them. The timeline starts when the organisation learns of the event, so robust intake is crucial.
- Significant Safety Issues (SSIs): The TGA requires 72-hour reporting of SSIs, which aligns closely with EMA standards. This demands 24/7 monitoring and quick escalation.
- Other Safety Issues (OSIs): OSIs must be assessed and reported within 30 days. The QPPVA must ensure a clear distinction between SSIs and OSIs for compliance.
- Product Information: The QPPVA should ensure timely updates to PI and CMI when new safety data emerges, linking pharmacovigilance with regulatory affairs.

The Non-Negotiable Residency Requirement
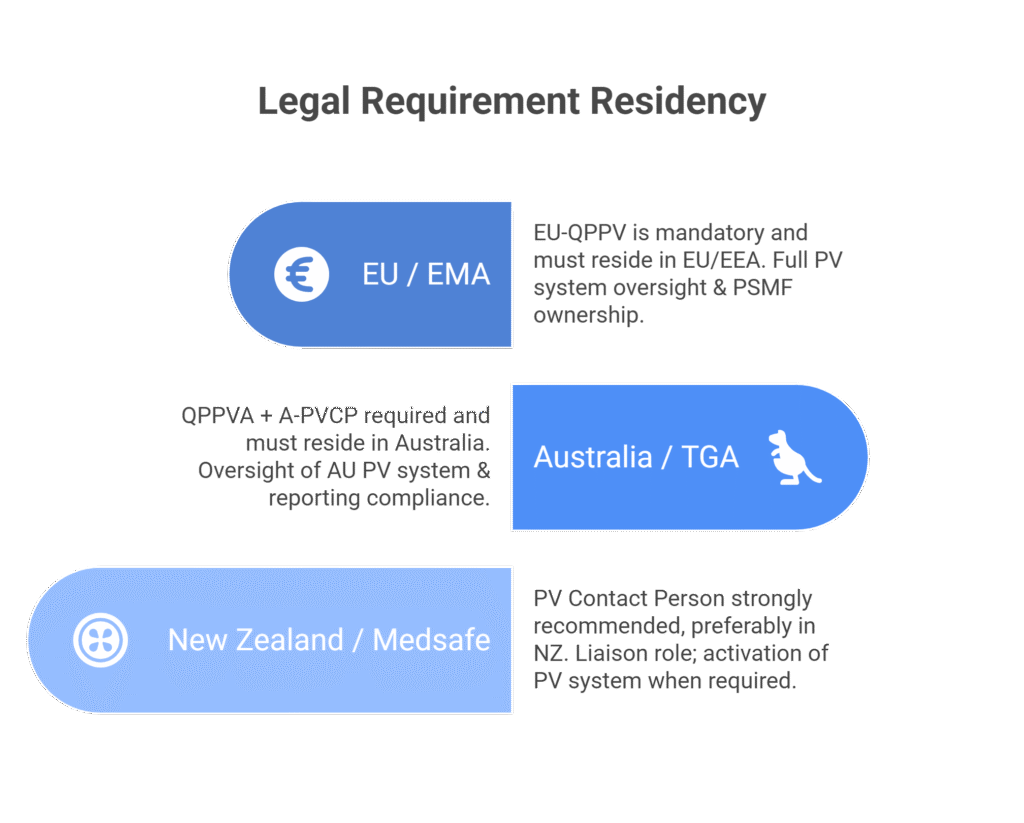
A key requirement is that the nominated Australian QPPVA and/or A-PVCP must reside in Australia, and that any deputy must also be based locally. This ensures accountability and accessibility to the TGA. As a result, this role cannot be managed remotely from overseas or by appointing an EU-QPPV for Australia.
This residency requirement influences organisational structure by necessitating dedicated Australian expertise and preventing consolidation of regional roles under a single individual based elsewhere. Plan budgets and resources accordingly when establishing your Australian PV function.
Qualifications: Medical Expertise and Regulatory Knowledge
Medical qualifications are not mandatory for the QPPVA role. However, the TGA strongly recommends that the QPPVA has access to a medically qualified person for clinical assessments. Ideally, this expert should reside in Australia and hold local registration to ensure relevant clinical judgment.
In addition to medical access, the QPPVA must demonstrate extensive training and experience in Australian pharmacovigilance regulations and practices. This expertise is essential for navigating the TGA’s specific requirements, which differ from those of the EU and New Zealand.
New Zealand: PV Contact Person vs. QPPVA Responsibilities
Medsafe’s Flexibility: Strong Recommendations vs. Legal Mandates
New Zealand’s approach is notably different from Australia’s. The Pharmacovigilance Guideline in New Zealand states that Medsafe “strongly recommends” sponsors nominate a PV contact person. This language indicates a guidance-based framework rather than a strict legislative mandate.
However, this flexibility does not equate to leniency. Core PV obligations remain mandatory. Medsafe expects robust systems for collecting, reviewing, and reporting safety information. Flexibility applies to the structure of accountability, not to compliance itself.
Role Definition: Liaison Over System Ownership
The New Zealand contact person primarily acts as a liaison between the PV system and Medsafe. They are not required to perform daily PV activities, but must be able to represent and activate the PV system when Medsafe requests information or action.
This model means Medsafe relies on the strength of your global PV infrastructure. Compliance failures in New Zealand often indicate broader systemic issues rather than isolated local lapses. Ensure your global processes fully support New Zealand’s requirements.
Location and Availability: Practical Accessibility
Medsafe prefers the contact person to be located in New Zealand. If not, they must at least be contactable during normal New Zealand business hours. This flexibility can create operational efficiencies.
Many sponsors appoint their Australian-resident QPPVA as the New Zealand contact person. This approach meets Medsafe’s accessibility requirement, maximises expertise, and maintains regional consistency. Ensure this individual is available during New Zealand business hours and understands Medsafe’s requirements.
Reporting Timelines: Harmonisation with Australia
Recent updates to the GRTPNZ (Edition 3.0, effective July 2024) have aligned New Zealand’s safety issue reporting with Australia and the EU. Significant Safety Issues must now be reported within 72 hours, and Other Safety Issues within 30 calendar days. This harmonisation simplifies regional compliance but requires consistent processes across jurisdictions.
European Union: EU-QPPV vs. QPPVA Responsibilities
The Legal Mandate: Central Authority and 24/7 Accountability
The EU operates the most prescriptive pharmacovigilance framework globally. Under Directive 2001/83/EC and the Good Pharmacovigilance Practices (GVP) modules, every Marketing Authorisation Holder (MAH) must have a Qualified Person for Pharmacovigilance “permanently and continuously at his disposal.” This isn’t optional—it’s a fundamental condition of holding EU marketing authorisation.
The EU-QPPV must be available 24 hours a day, 7 days a week. This requirement reflects the QPPV’s role as the single point of contact for the EMA and national authorities. Robust deputy arrangements and escalation protocols are necessary to ensure continuous coverage.
Expansive Scope: PSMF Oversight and Global Responsibility
The EU-QPPV is ultimately responsible for your entire pharmacovigilance system. Critically, they maintain oversight of the Pharmacovigilance System Master File (PSMF)—a comprehensive, living document describing your PV system for EU-marketed products.
The QPPV’s responsibilities extend globally when products have worldwide distribution. Specifically, the PSMF must reflect the worldwide availability of safety information, positioning your EU-QPPV as the natural hub of your global PV architecture. Consequently, this individual needs visibility into safety data from all markets, not just European ones.
Residency and Qualification: The Highest Bar
The EU-QPPV must reside and perform their duties within the European Union or European Economic Area (Norway, Iceland, Liechtenstein). This ensures compliance with EU law and accessibility to regulators. The PSMF must also be located where main PV activities occur or where the QPPV operates, within the EU/EEA.
GVP Module I specifies that the QPPV should have pharmacovigilance system management skills and expertise in medicine, pharmaceutical sciences, epidemiology, or biostatistics. If the QPPV lacks medical training, assistance from a medically trained person is required, with this arrangement formally documented in the PSMF.
Comparative Analysis: Strategic Implications for Global PV Architecture
Legal Mandate and Authority: A Spectrum of Requirements
The three frameworks create a spectrum of legal authority:
- European Union: The EU-QPPV holds legally mandated, personally accountable responsibility for the entire PV system with broad authority over quality systems and PSMF.
- Australia: The QPPVA operates under a recommended mandate and also serves as the “contact person”. but functions within a QPPV-level system of oversight due to regulatory expectations and industry standards.
- New Zealand: The contact person is “strongly recommended” rather than legally mandated, functioning primarily as an accessible liaison to the sponsor’s PV system.
Residency Requirements: Shaping Organisational Structure
Residency rules significantly affect operational structure. Australia’s strict residency requirement prevents remote management from overseas. The EU’s rule centralises authority within the Union. New Zealand’s flexibility allows the Australian QPPVA to serve both markets when appropriately structured.
These differences often lead multinational companies to adopt a “Hub-and-Spoke” model. The EU-QPPV serves as the central hub, establishing core quality systems and standards. The Australian QPPVA serves as the primary spoke, implementing global processes tailored to TGA requirements. The Australian QPPVA can also serve as New Zealand’s sub-spoke, maximising expertise and meeting Medsafe’s accessibility requirements.
Key takeaways
Core Competencies of an Effective PV Leader
- Regulatory knowledge: Stay current on TGA, EU, and NZ requirements, as well as broader trends, for practical risk assessment and regulatory communication.
- Quality systems: Develop SOPs, manage CAPAs, deliver training, and ensure audit readiness to keep systems compliant and robust.
- Analytical skills: Interpret complex data, identify safety signals, assess causality, and implement risk mitigation.
- Leadership: Guide teams, influence stakeholders, and communicate clearly with regulators, management, and healthcare professionals.
Practical Implementation – “5 Steps to Strengthen Your Local PV Representation.
- Document Authority: Define the QPPVA or Contact Person’s authority within your PV system and ensure this is reflected in job descriptions, SDEAs, and organisational charts.
- Build Redundancy: Assign and document deputies for 24/7 coverage; regulators assess continuity of oversight.
- Train for Readiness: Conduct regular QPPVA/Contact Person training on TGA, EMA, and Medsafe reporting timelines and SSI definitions.
- Review SDEAs: Ensure every partner agreement defines escalation pathways and reporting responsibilities.
- Test Inspection Readiness: Conduct mock inspections quarterly — focusing on interview readiness, documentation traceability, and decision-making evidence.
Final word
Pharmacovigilance leaders are vital for safe, compliant operations. While requirements vary—EU QPPV, Australian QPPVA, New Zealand contact person—all must be highly competent and accountable for safety. Meeting diverse frameworks requires a robust global PV system. The “Hub-and-Spoke” model aligns the needs of the EU, Australia, and New Zealand while enhancing efficiency.A PV system’s success hinges on leadership expertise and authority. Choose experienced leaders and support them with resources and a culture of safety. As regulations and products grow more complex, strong PV leadership is essential for compliance, governance, and patient safety.
References & Further Learning
Australian Regulatory Guidance
- Therapeutic Goods Administration. (2023). Pharmacovigilance responsibilities of medicine sponsors.
- Retrieved from Therapeutic Goods Administration. Pharmacovigilance Inspection Program.
New Zealand Regulatory Guidance
European Union Standards
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) – Module I: Pharmacovigilance systems and their quality systems.
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) – Module II: Pharmacovigilance system master file (Rev 2).
Disclaimer: This content is for general information only and does not constitute regulatory, legal, or professional advice. Circumstances vary—consult qualified regulatory professionals for guidance specific to your organisation and jurisdiction.
This educational guide reflects current international standards, regulatory guidance, and industry best practices as of the published date.
© 2025 GxPVigilance. All rights reserved.
Need support setting up your local PV contact system?
- Our GxPVigilance team helps sponsors establish and validate inspection-ready pharmacovigilance systems — from QPPVA/A-PVCP appointment to signal management oversight.
- Contact us or book a Consultation to discuss how we can help streamline your compliance workflow.




